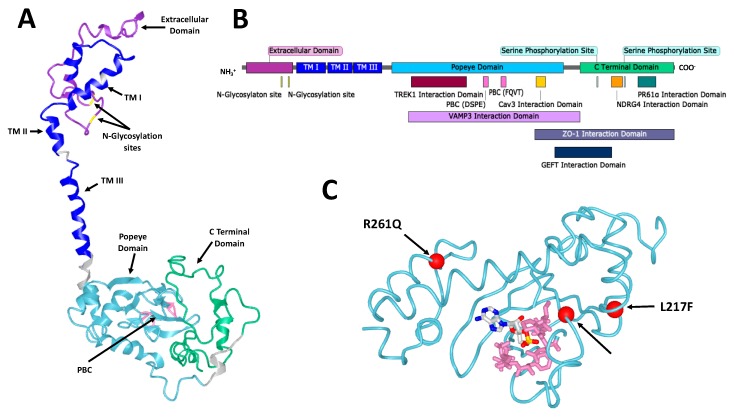
Figure 1.
The structure of Popeye domain containing (POPDC) proteins. (A) A homology model of human POPDC1. POPDC1 shares a similar structure with POPDC2 and POPDC3. Some features are indicated including the extracellular domain (purple), the two Asn residues of the N-glycosylation sites (yellow), the three transmembrane (TM) domains (blue), the Popeye domain (cyan), the DSPE and FQVT motifs, which are part of the phosphate binding cassette (PBC, pink) and the C-terminal domain (green). The model was produced using the Phyre2 algorithm [16]. (B) A linear map of POPDC1. Structural features are indicated as well as the sites of interaction of multiple interaction partners. Many of the interaction sites are approximate and have not been precisely identified. (C) A homology model of the Popeye domain of human POPDC3, shown with cyclic adenosine monophosphate (cAMP) in its predicted binding site. The DSPE and FQVT motifs of the PBC are shown in pink. The positions of the three pathological mutations in POPDC3 reported by Vissing et al. [17] are shown as red spheres. The model was produced using the Phyre2 algorithm and the cAMP binding site was predicted using the 3DLigandSite predictor [16,18].