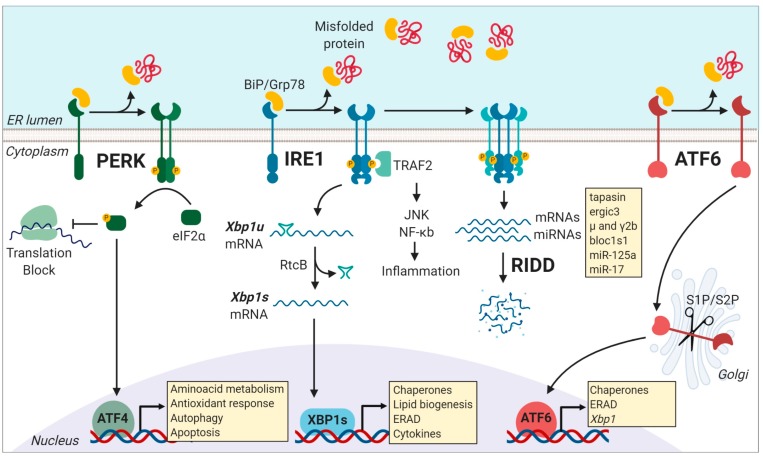
Figure 1.
Activation of the three unfolded protein response (UPR) pathways is initiated by misfolded protein accumulation in the endoplasmic reticulum (ER). Phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α) is dependent of the PKR-like kinase (PERK). This process inhibits ribosome assembly, which causes a translational block allowing the cell to cope with temporary ER-stress. However, ATF4 escapes this translation inhibition under conditions of stress and induces the transcription of genes related to cell survival including those involved in compensatory autophagy. Activation of the endoribonuclease domain of IRE1 is caused by the dissociation of the binding immunoglobulin protein (BiP) from the luminal domain of IRE1, causing the non-conventional splicing of the unspliced form of the X-box binding protein 1 (xbp1u) mRNA to produce xbp1s mRNA, which encodes the potent transcriptional activator, XBP1s. Among the various targets of XBP1s are genes encoding for chaperones, genes that assist in the degradation of misfolded proteins via ER-associated degradation (ERAD), lipid biogenesis, and cytokine production. Under conditions of chronical stress, IRE1 is hyper-activated, and it cleaves additional RNAs, such as mRNAs and miRNAs, through a process called Regulated IRE1 dependent decay (RIDD). After BiP dissociation from ATF6 during ER stress, ATF6 travels to the Golgi compartment, where it is processed by the S1P/S2P enzymes. The processed ATF6 fragment functions as a transcription factor that enhances protein folding at the ER level and also promote the expression of target genes that assist in degradation processes, including ERAD. Figure created with Biorender.com.