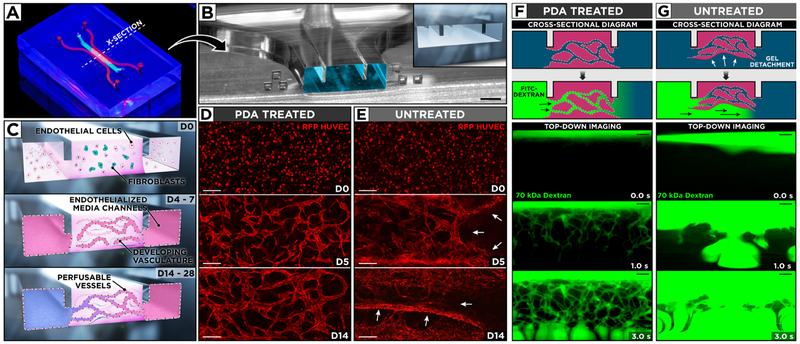
Figure 4.
(A) Photograph of our microengineered device for generation and extended culture of perfusable human blood vessels. (B) The microchannel cross-section along the dotted line in (A) shows the protruding barriers designed to pin hydrogel infused into the central channel. Scale bar: 500 μm. (C) Vascular endothelial cells and fibroblasts are co-cultured in a central hydrogel scaffold created by capillary pinning between two endothelialized media channels for over 14 days to form a 3D network of self-assembled and perfusable blood vessels lined with living endothelial cells. (D, E) Confocal micrographs of vascular development during 14-day culture of red fluorescent protein-expressing HUVECs and human lung fibroblasts. PDA treatment of the microchannels yields stable hydrogels with well-defined 3D vasculature, whereas untreated devices suffer from hydrogel collapse and the resultant loss of vascular architecture. White arrows indicate areas of gel detachment. Scale bars: 200 μm. (F) In PDA-coated devices, the engineered vessels become perfusable as demonstrated by the flow of 70 kDa FITC–dextran through their intraluminal compartments. (G) In contrast, the injected fluorescent dye flows beneath and around cell-laden scaffolds in untreated devices as a result of gel detachment. Scale bars: 200 μm.