Figure 10.
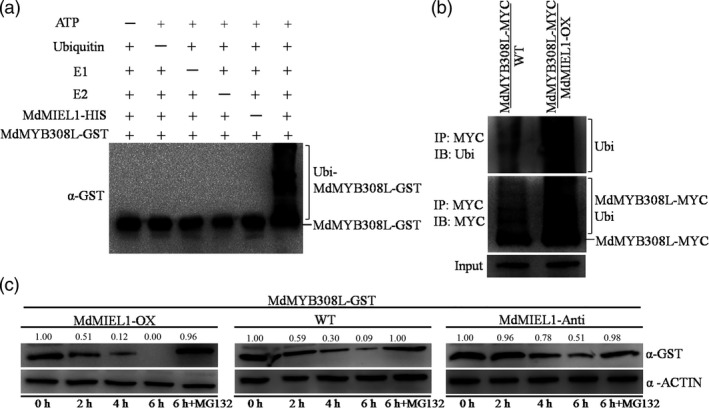
MdMYB308L is an ubiquitination substrate of MdMIEL1. (a) MdMIEL1 ubiquitinates MdMYB308L in vitro. MdMIEL1‐HIS was tested for E3 ubiquitin ligase activity in the presence and absence of ATP, ubiquitin, E1, E2, MdMIEL1‐HIS and MdMYB308L‐GST. The protein gel blot was analysed using a GST antibody. (b) MdMIEL1 ubiquitinates MdMYB308L in vivo. MdMYB308L‐MYC was immunoprecipitated using MYC antibody from the two transgenic apple calli (MdMYB308L‐MYC and MdMYB308L‐MYC/MdMIEL1‐OX). Immunoblotting using an ubiquitin antibody was shown on the top and using a MYC antibody at below. (c) MdMIEL1 promotes the degradation of MdMYB308L‐GST protein in vitro. Total proteins extracted from wild‐type and transgenic apple calli with or without 100 μm MG132 treatment were incubated with the purified MdMYB308L‐GST fusion protein. The samples were collected at the indicated time. ACTIN was used as internal reference. The relative intensity ratio between the GST and the ACTIN was shown.
