5.
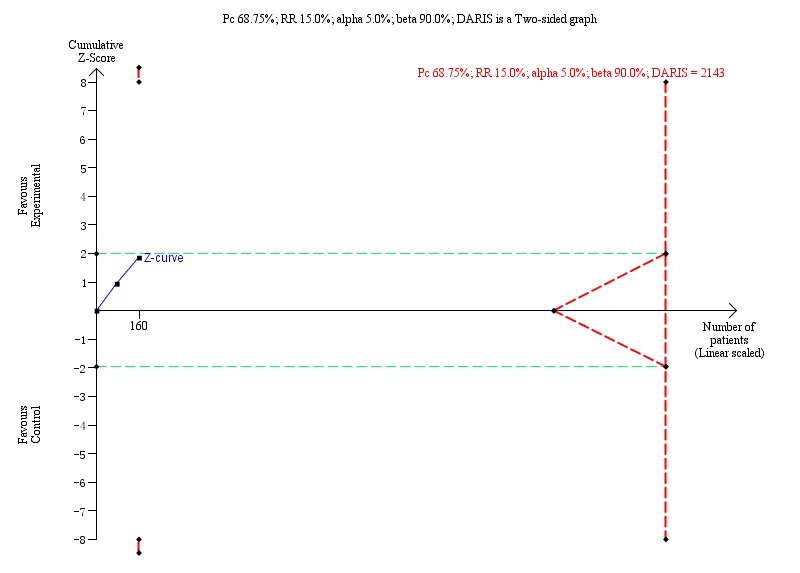
Proportion of participants with detectable HBeAg: Trial Sequential Analysis (risk ratio (RR) random‐effects model) including randomised clinical trials comparing Xiao Chai Hu Tang formula versus no intervention for people with chronic hepatitis B. The pair‐wise meta‐analysis includes 2 trials with 160 participants and found a RR of 0.72 (95% CI 0.17 to 3.02). The Trial Sequential Analysis was made with event proportion in the control group 68.75%, relative risk reduction 15%, alpha 5.0%, power 90%, and model‐based diversity 57%. The Trial Sequential Analysis‐adjusted CI was 0.17 to 3.02.
