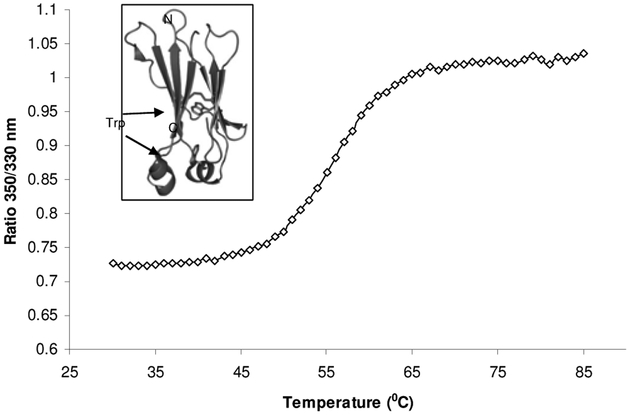
Fig. (6).
Temperature induced unfolding of the recombinant human IgG1 CH2 domain. The graph was obtained using Avacta Optim1000 instrument. The ratio of 350 nm/330 nm indicated that the two tryptophan residues in CH2 were exposed as the protein unfolded at 50–60 °C. The unfolding transition thermal mid points (Tm) was calculated to be 56.2 °C. The two tryptophan residues in the CH2 domain are buried inside the folded protein as seen in the crystal structure [26], inset of the figure.