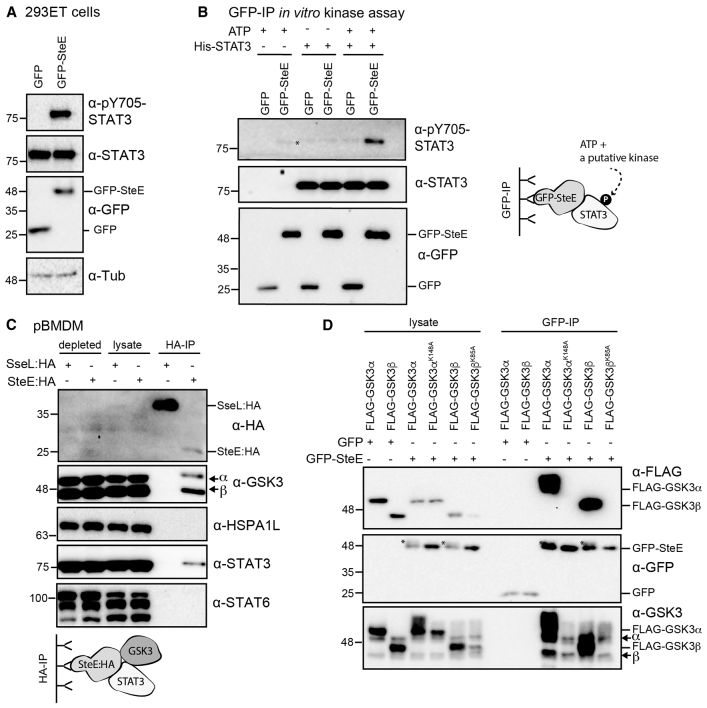
Figure 2.
SteE Interacts with Catalytically Active GSK3 and STAT3
(A) Whole-cell lysates from 293ET cells expressing GFP or GFP-SteE were analyzed by immunoblot with antibodies against STAT3, pY705-STAT3, GFP, and tubulin (Tub) as a loading control. Data are representative of three independent experiments.
(B) GFP or GFP-SteE was expressed in 293ET cells, immunoprecipitated, and assessed for its ability to phosphorylate exogenously added recombinant His-STAT3 in an in vitro kinase assay. The ∗ represents endogenous phosphorylated STAT3 that was detected upon immunoprecipitation with GFP-SteE but not with GFP. Data are representative of three independent repeats.
(C) pBMDMs were infected with steE mutant Salmonella carrying pWSK29-SteE:HA or sseL mutant Salmonella carrying pWSK29-SseL:HA. 17 h after uptake, HA-tagged effectors were immunoprecipitated from cell lysates and assessed for their ability to bind endogenous GSK3, STAT3, STAT6, or HSPA1L as indicated. Immunoblots are representative of three independent experiments.
(D) GFP or GFP-SteE, expressed in 293ET cells, was immunoprecipitated and assessed for its ability to interact with the indicated co-expressed FLAG-tagged GSK3 variants and endogenous GSK3 (indicated with arrows). Data are representative of three experiments. The ∗ indicates a higher-molecular-weight form of SteE.