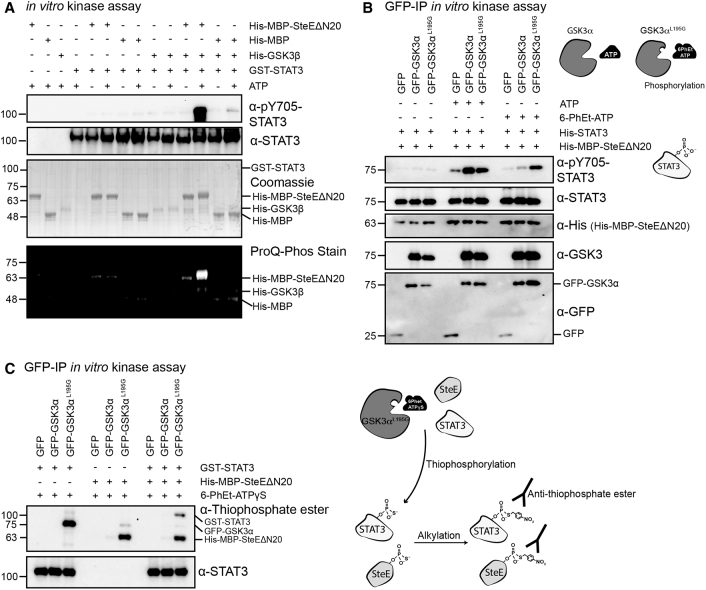
Figure 5.
SteE Enables GSK3 to Phosphorylate STAT3
(A) In vitro kinase assays containing 5 μg recombinant His-GSK3β, 12.5 μg His-MBP or His-MBP-SteEΔN20, and 0.4 μg GST-STAT3, all with or without 1 mM ATP, were assayed by immunoblot, Coomassie stain, and Pro-Q Diamond phosphoprotein stain. Data are representative of three repeats.
(B) GFP, WT GFP-GSK3α, and GFP-GSK3αL195G were expressed in 293ET cells, immunoprecipitated, and assessed by immunoblot for their ability to phosphorylate exogenously added recombinant His-STAT3 in an in vitro kinase assay containing His-MBP-SteEΔN20 and either no ATP, ATP, or 6-PhEt-ATP. Data are representative of three experiments.
(C) In vitro kinase assays containing immunoprecipitated GFP, WT GFP-GSK3α or GFP-GSK3αL195G; and 2 μg GST-STAT3 and/or 1.6 μg His-MBP-SteEΔN20, as indicated were incubated with N6-PhEt-ATPγS as the phosphate donor. Samples were assayed by immunoblot with the indicated antibodies. Data are representative of two experiments.