Figure 1.
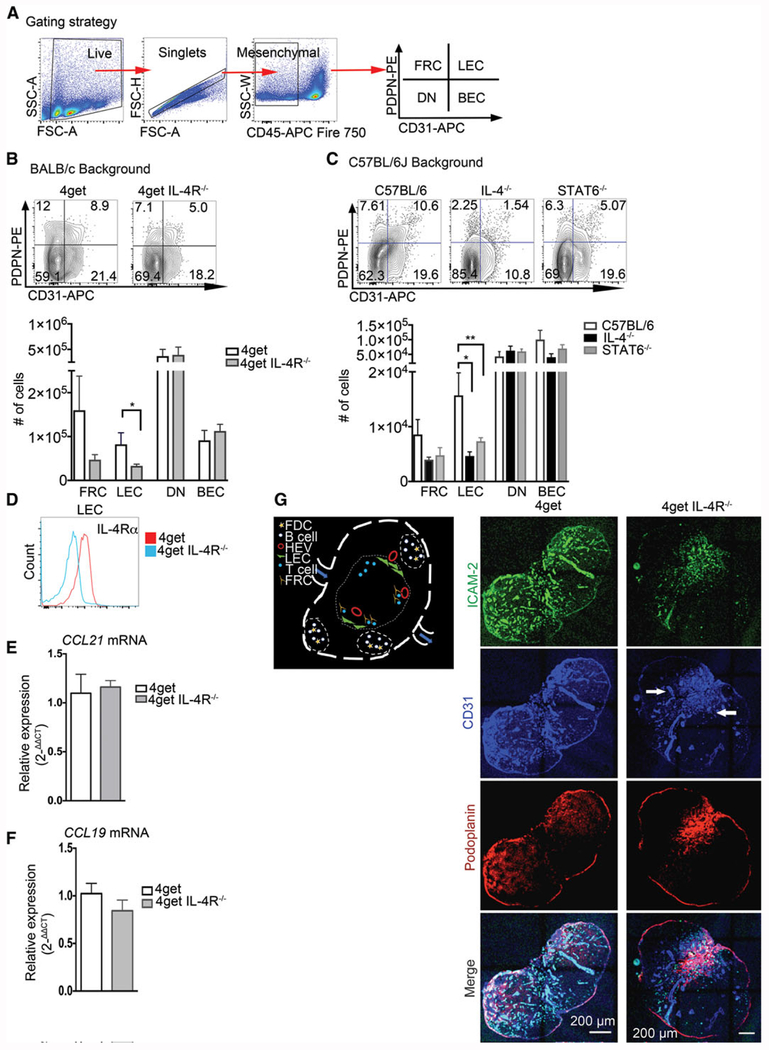
Maintenance of endothelial cells in peripheral lymph nodes in steady state requires IL-4 signaling. C57BL/6J, IL-4KO, STAT6KO, 4get homozygous, and 4get IL-4RαKO mice were sacrificed at 6–8 weeks of age, and popliteal lymph nodes were collected, digested, and single cell suspensions were obtained and analyzed by flow cytometry for expression of indicated markers. Alternatively, intact popliteal lymph nodes were collected, frozen in optimum cutting temperature (OCT) medium, sectioned, and tile scans were acquired with a laser scanning confocal microscope. (A) Gating strategy for lymphatic endothelial cells (B) Popliteal lymph node lymphatic endothelial cells from CD45− population (defined as PDPN+CD31+), BEC (defined as CD31+PDPN−), FRC (PDPN+, CD31−), and DN in 4get homozygous (control) and 4get IL-4RKO mice and (C) in C57BL/6J (control) and IL-4KO and STAT6KO in popliteal lymph node, error bar denotes mean ± SEM. (D) Expression of IL-4Rα in lymphatic endothelial cells from popliteal lymph node determined by flow cytometry. (E and F) Relative expression of CCL21 and CCL19 by quantitative PCR, respectively from naïve popliteal lymph nodes normalized to naïve 4get (control), bars show mean ± SEM. (G) Schematic of lymph node showing cells of interest in B-cell follicles and T-cell area. (H) Popliteal lymph node cryosections were stained for ICAM-2, green; CD31, blue and podoplanin, red. Scale bar: 200 μm. Arrowhead denotes CD31+ endothelial cells clusters preferentially localized in the medulla. Student t-test was used to determine statistical significance *p < 0.05, **p < 0.01. FACS data shown are concatenated from 3–5 mice per group and experiments were performed 3–4 times. Confocal images are representative of three different experiments with three mice per group.