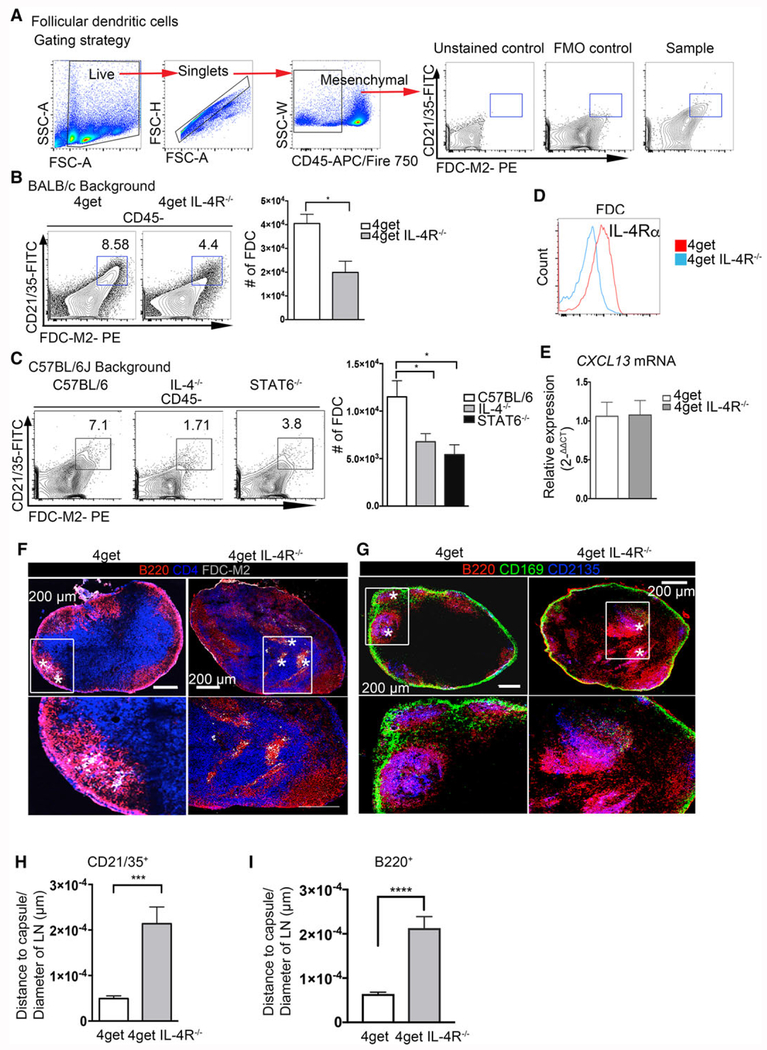
Figure 2.
IL-4 is required for proper follicular dendritic cell and B-cell follicle organization in peripheral lymph nodes in steady-state. Lymph nodes from naïve C57BL/6J, IL-4KO, STAT6KO, 4get homozygous, and 4get IL-4RαKO mice were digested with collagenase and smashed through a cell strainer to be analyzed by flow cytometry or frozen in OCT medium, and tile scans of cryosections were acquired with a laser scanning confocal microscope. (A) Gating strategy and staining controls. (B) Follicular dendritic cells (CD21/35+ FDC-M2+) gated from CD45− obtained from 4get homozygous and 4get IL-4Rα KO popliteal lymph nodes and (C) popliteal lymph nodes of C57BL/6J, IL-4KO, and STAT6KO mice. (D) Expression of IL-4Rα in follicular dendritic cells from naïve popliteal lymph node. (E) Relative expression of CXCL13 from naïve pLNs, normalized to naïve 4get (control) bars show mean ± SEM. (F) Frozen popliteal lymph node sections from 4get homozygous (control) and 4get IL-4RαKO mice were stained for B220 (red), CD4 (blue), and FDC-M2 (gray). (G) Sections were stained for CD21/35 (blue, follicular dendritic cells), B220 (red, B cells), and CD169 (green, subcapsular macrophages). Asterisk denotes FDC-M2+ cells and CD21/35+ cells, scale bar: 200 μm. (H) Quantification of the distance of CD21/35+ FDC to the LN capsule normalized to the LN diameter. (I) Quantification of B220+ cells from the lymph node capsule and normalized to the total lymph node area. Bar depicts mean ± SEM. Image is representative of two independent experiments with 3–4 mice per group. FACS data shown are representative from 3–5 mice per group from three independent experiments. Confocal microscopy images are representative of 3–4 mice per group of three independent experiments. *p < 0.05, ***p < 0.001; statistical significance was calculated by unpaired Student’s t-test.