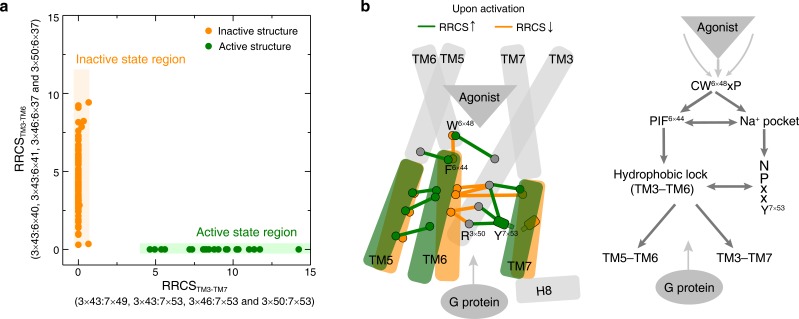
Figure 5. Common activation model of class A GPCRs reveals major changes upon GPCR activation.
(a) Active and inactive state structures form compact clusters in the 2D inter-helical contact space: RRCSTM3-TM7 (X-axis) and RRCSTM3-TM6 (Y-axis). GPCR activation is best described by the outward movement of TM6 and inward movement of TM7, resulting in switch in the contacts of TM3 from TM6 to TM7. (b) Common activation model for class A GPCRs. Residues are shown in circles, conserved contact rearrangements of residue pairs upon activation are denoted by lines.