Fig. 6. Functionality of nano-SCE as Li-ion electrolyte.
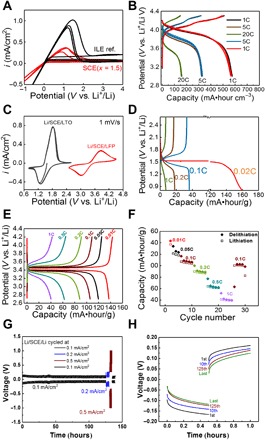
(A) Cyclic voltammogram of nano-SCE (x = 1.5, as synthesized after vacuum drying) (red) and ILE reference (black) measured in three-electrode configuration with Li as working, counter, and reference electrodes (SEI resistance estimated from IR drop on cathodic current is 0.9 and 1.8 kilo-ohm·cm2 for ILE and SCE, respectively). (B) Galvanic charge/discharge curves of Li/SCE (x = 1)/100-nm thin-film LiMn2O4 cell for five cycles at C-rates of 1C, 5C, and 20C. (C) Cyclic voltammograms of the Li/SCE/40-μm Li4Ti5O12 and Li/SCE/30-μm LiFePO4 powder electrode cells (1 mV/s). (D) Galvanic charge/discharge curves of Li/SCE/40-μm Li4Ti5O12 powder electrode at 1C, 0.1C, 0.2C, and 0.02C. (E) Galvanic charge/discharge curves of the Li/SCE/30-μm LiFePO4 powder electrode at 1C, 0.5C, 0.2C, 0.1C, 0.05C, and 0.01C. (F) Capacity (filled diamonds for delithiation and open squares for lithiation) versus cycle number of the Li/SCE/30-μm LiFePO4 powder electrode; the thickness of the SCE in the cells is about 280 μm. The density of the LFP and LTO cathode is about 1.9 and 11.0 mg/cm2, respectively. (G) Potential versus time curves of a Li/SCE/Li stack cycled at current densities of 0.1, 0.2, 0.5, and 0.1 mA/cm2. (H) The 1st, 10th, 125th, and last polarization of the Li/SCE/Li stack stressed at 0.1 mA/cm2, shown in (G). For (G) and (H), the SCE has a conductivity of 0. 34 mS/cm, and the thickness of the SCE pellet is 0.152 cm.
