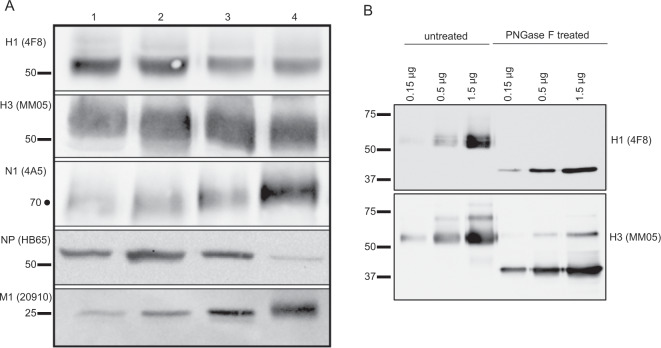
Fig. 1. Viral protein composition of licensed seasonal influenza vaccines.
In a, licensed seasonal influenza vaccines Fluzone 2014–15 (Lane 1), Fluzone 2015–16 (Lane 2), FluLaval 2013–14 (Lane 3), and FluLaval 2015–16 (Lane 4) were surveyed for the presence of H1 (55 kDa), H3 (55 kDa), NA (75 kDa), NP (56 kDa), and M1 (27 kDa) using antibodies listed in Supplementary Table 2 and indicated to the left. Vaccines were prepared to a final concentration of 2.7 µg of total HA per sample for immunoblot, based on manufacturer-reported hemagglutinin quantity. The proteins within the vaccines were fractionated by SDS-PAGE gel and then analyzed by western blot as described. In b, Fluzone proteins in the 2017–18 vaccine were treated with the enzyme PNGase-F to remove any N-linked glycans present on the HA proteins and then probed for H1 and H3. Untreated vaccines, applied at varying concentrations of HA, are shown on the left and the PNGase-treated vaccines are shown on the right. Molecular weight markers are indicated. A dot represents a molecular weight extrapolated from the mobility of the molecular weight markers.