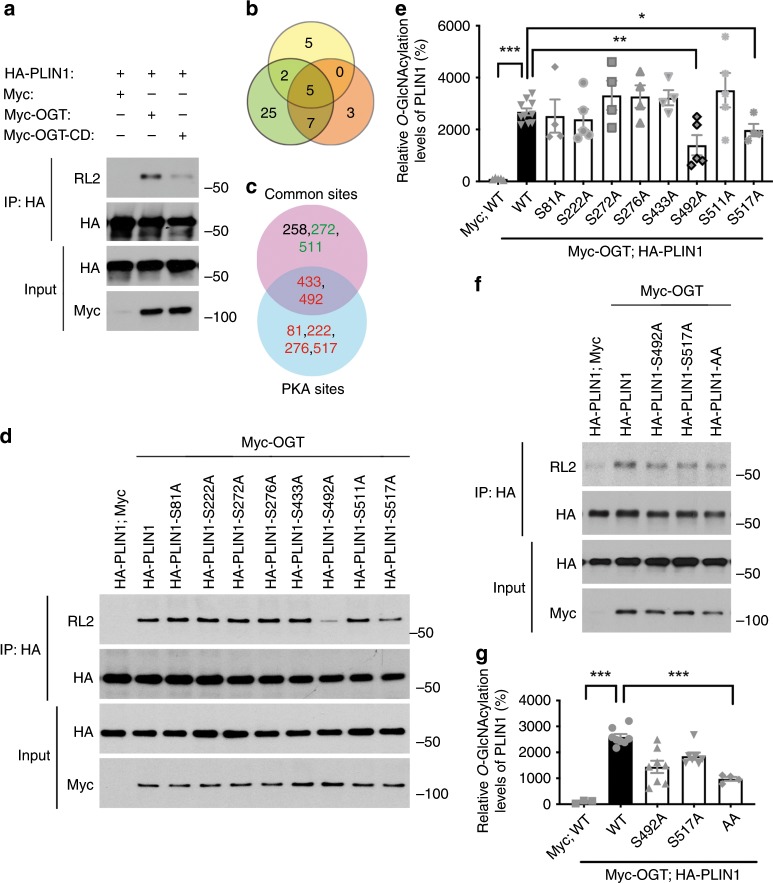
Fig. 5. OGT catalyzes PLIN1 O-GlcNAcylation.
a Immunoprecipitation (IP) and Western blot analysis showing that OGT overexpression enhances PLIN1 O-GlcNAcylation and the catalytic dead mutation of OGT greatly impairs its ability to catalyze PLIN1 O-GlcNAcylation; RL2 is an antibody against O-GlcNAc moieties. b Overlapping of predicted O-GlcNAcylation sites determined by three independent analyses. c Final candidate sites, known PKA phosphorylation sites (red colored) were included, common sites from b that are adjacent to the PKA sites (green colored) were also included. d, e IP, Western blot analysis, and quantification results showing that OGT catalyzes PLIN1 O-GlcNAcylation primarily at serine 492 and serine 517 (n = 10 for WT positive control, 4–5 for other groups). f, g IP, Western blot analysis, and quantification results showing that serine 492 to alanine and serine 517 to alanine double mutation in PLIN1 (PLIN1-AA) largely eliminated its overall O-GlcNAcylation (n = 8 for WT positive control, 3–8 for other groups); data are presented as mean ± s.e.m. Statistical analysis: ANOVA with Dunnett’s multiple comparisons, *p < 0.05, **p < 0.01, and ***p < 0.001. Source data are provided as a Source Data file.