Abstract
INTRODUCTION
Placebo effects in human clinical trials for depression treatment are robust and often comparable to drug effects. Placebo effects are traditionally difficult to study in rodents due to the slow-onset action of classical antidepressant drugs. We hypothesized that the rapid antidepressant actions of ketamine would allow modeling antidepressant placebo effects in rodents.
METHODS
Male and female CD-1 mice received either ketamine or saline injections with concomitant exposure to specific environmental conditioning stimuli, for a total of three drug/conditioning sessions each 2 weeks apart. Two weeks later, during an evocation phase, mice were exposed to the drug-paired conditioning stimuli or no conditioned stimuli followed by testing for motor stimulatory actions and antidepressant-like effects using the forced swim test. Negative (no ketamine administration at any time) and positive (acute ketamine administration prior to evocation testing) control groups were included as comparators.
RESULTS
Both male and female mice exhibited increased locomotor activity following ketamine administration during the conditioning phase, which was not observed following exposure to the conditioning stimuli. Exposure to the conditioning stimuli previously paired with ketamine, similar to an acute ketamine administration, reduced immobility time in the forced swim test both 1 and 24 h after administration in male, but not female, mice.
CONCLUSIONS
These results represent the first evidence of antidepressant-like placebo-conditioned effects in an animal model. The developed approach can be used as a model to explore the neurobiological mechanisms of placebo effects, their possible sexually dimorphic effects, and relevance to mechanisms underlying antidepressant action.
Keywords: ketamine, placebo, antidepressant, classical conditioning, depression, animal model, mice, biological sex
INTRODUCTION
Placebo effects occur following administration of inert substances given with verbal suggestions explicitly stating the benefits of the compound, via social learning, or through cued and contextual conditioning (Colagiuri et al. 2015; Colloca et al. 2013; Peiris et al. 2018). While placebo effects are typically viewed as a hindrance when distinguishing drug specific from non-specific effects for medication development and regulatory approval purposes, these effects could also be considered beneficial in clinical practice, since they can positively impact treatment outcomes (Andrews 2001). Placebo effects are consistently robust in clinical trials of pharmacological depression treatment (Fournier et al. 2010; Kirsch et al. 2008; Kirsch and Sapirstein 1998), with average placebo response rates ranging between 35 and 40% (Furukawa et al. 2016). The clinical relevance and large size of placebo antidepressant effects, independent of direct effects of treatment themselves, suggest that the relevant mechanisms could be exploited for more effective therapies.
Some animal models for the study of placebo effects have been developed, and used primarily in preclinical research on analgesia (Colloca 2019; Guo et al. 2010; Keller et al. 2018; Zhang et al. 2013). In these studies, an active analgesic compound, such as morphine, is paired with a conditioning stimulus (CS), and later the inert CS is delivered to rats or mice in the absence of any exogenous compound administration to produce analgesia (Guo et al. 2010; Zhang et al. 2013). However, such approaches have not been successful in antidepressant placebo research, inherently due to the fact that classical conditioning requires brief temporal delays between the CS and the effect elicited by the unconditioned stimulus, whereas typical antidepressants have a slow therapeutic onset time of weeks to months (Rush et al. 2006). In contrast to the slow therapeutic onset of classical, monoamine-based, antidepressant medications, the anesthetic ketamine, exerts rapid (often within 2 h) antidepressant effects when administered at sub-anesthetic doses in depressed (Berman et al. 2000) and treatment-refractory depressed patients (Fava et al. In press; Lapidus et al. 2014; Murrough et al. 2013; Singh et al. 2016; Zarate et al. 2006).
Experimental blinding, along with randomization and allocation concealment, are critical when assessing effects directly attributable to ketamine against regression to the mean, the natural variation in the course of the symptoms expectancy, and placebo effects or other non-specific effects. A recent integrative analysis of nine previous clinical trials examined the effect of midazolam versus saline as an approach for preserving blinding in ketamine studies. The antidepressant effect of ketamine, while significant, was smaller when compared with midazolam than when the comparator was saline (Wilkinson et al. 2019). This difference was driven by greater improvement in the midazolam compared to the saline group. It is possible that perceiving the drug administration (midazolam versus saline) made people believe that they received the active treatment therefore increasing placebo and expectancy effects (Wilkinson et al. 2019). Although human trials with ketamine presents the challenge of blinding study participants, one study indicates that both placebo and ketamine induce a strong decrease of the white matter in frontal and temporal regions bilaterally (Hoflich et al. 2017). Future studies directly comparing ketamine and placebo can help to identify commonalities between drug and placebo effects, as studied in the context of treatment for other diseases (Benedetti et al. 2005; Colloca 2019; Mayberg et al. 2002).
In rodent and clinical studies, a single ketamine administration at low, sub-anesthetic doses, exerts rapid and sustained antidepressant-like actions requiring only a single administration (Autry et al. 2011; Li et al. 2010; Maeng et al. 2008); also see Zanos and Gould, (2018). Therefore, we hypothesized that the rapid antidepressant actions of ketamine could be used for modeling antidepressant-like placebo effects. Here, during three exposures each two weeks apart, we paired ketamine administration with concomitant exposure to a CS. Mice were subsequently tested for placebo antidepressant-relevant responses following exposure to the CS alone.
METHODS
Animals
48 male and 48 female 7 week old CD-1 mice were purchased from Charles River Laboratories (Willington, MA, USA). Mice were separated by sex and housed four mice per cage, with a 12-hour light/dark cycle (lights on/off at 07:00/19:00). Mice were acclimated to the University of Maryland Baltimore animal vivarium for at least seven days prior to experimentation and had food and water available ad libitum. All experimental procedures were approved by the University of Maryland, Baltimore Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
(R,S)-ketamine HCl (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% saline and delivered intraperitoneally (i.p.) in a volume of 7.5 ml/kg of body mass. The dose used for ketamine (i.e., 10 mg/kg) was based on a previous preclinical report showing antidepressant-relevant behavioral changes using this dose in both male and female CD-1 mice (Zanos et al. 2016).
Experimental Conditions and Conditioning paradigm
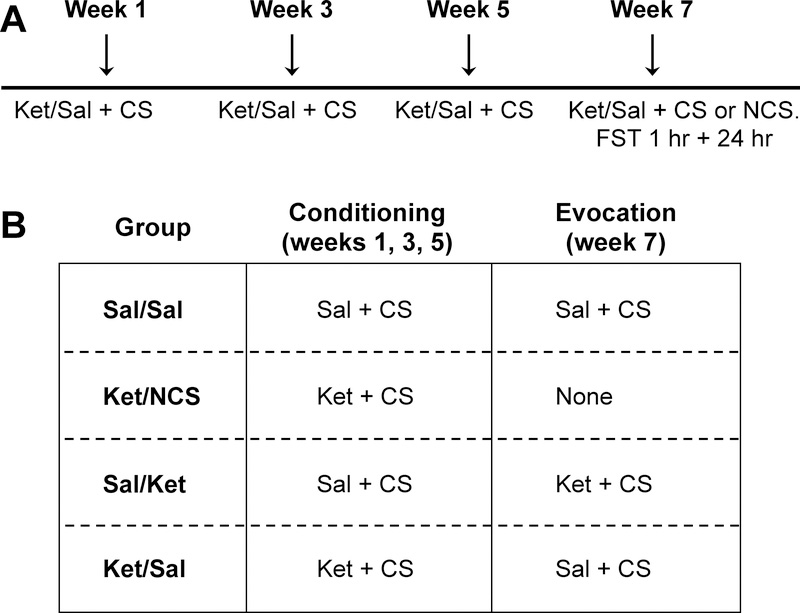
The total duration of the experiment was 7 weeks (for a timeline see Figure 1A) and included a conditioning and an evocation phase. During the conditioning phase, mice received an injection of ketamine or saline once every other week - i.e., weeks 1, 3 and 5 – and were concomitantly exposed to the CS. The CS consisted of an immediate (post-injection) 60-min exposure to an open-field arena containing ~0.5g sugar-free chocolate (milk chocolate miniature candy bars; The Hershey Company, Hershey, Pennsylvania, U.S.A.) placed in a corner under blue light conditions (10 lux intensity). Injections were separated by two weeks to avoid residual effects of ketamine, which have been reported to last up to a week post-injection in rodents (Autry et al. 2011; Li et al. 2010; Maeng et al. 2008). During the evocation period (week 7), the effects of the conditioning were examined under no-drug administration conditions. The CS was presented to all mice during the three conditioning sessions, and to 3 of the 4 groups during evocation (see Figure 1A).
Figure 1. Experimental overview.
Timeline of experiments (A). Mice were randomly allocated to 4 groups (B). There were two phases to experimentation. The first phase, i.e., the conditioning phase, occurred on weeks 1, 3, and 5. Conditioning involved pairing an injection (saline or 10 mg/kg ketamine) with the injection context. The second phase, the evocation phase, occurred during week 7. Evocation is when the effects of conditioning were tested in the absence of drug administration. CS=conditioning stimulus; Sal=saline; Ket=ketamine; NCS=no conditioning stimulus.
Mice were randomly allocated into the following four different experimental groups (Figure 1B), with each group comprised of one mouse from each cage:
(i) Sal/Sal received a saline injection during each conditioning session and a saline injection during the evocation session (negative control group).
(iii) Ket/no CS (Ket/NCS) received ketamine during each of the conditioning sessions and no CS during the evocation phase. During the evocation session (week 7), when the cage-mates were re-exposed to the CS, Ket/NCS mice remained single housed for 1 hour in a new cage with access to normal chow. This group served to examine any possible carry-over effects of the prior three injections of ketamine.
(ii) Sal/Ket received a saline injection during each conditioning session and ketamine during the evocation session (positive control group for the acute effects of ketamine).
(iv) Ket/Sal received ketamine during each of the conditioning sessions and saline during evocation session (placebo group). The Ket/Sal group functioned as the placebo conditioned group, in that any behavioral effects observed would be due to exposure to the CS previously paired with ketamine.
Experimenters were blind to treatment allocation.
Open-field Test (OFT)
Following each drug administration, mice were placed into individual open-field arenas (50 cm length × 50 cm width × 38 cm height; San Diego Instruments, San Diego, CA, USA) for 60 minutes. Locomotor activity was assessed during this time and distance travelled was analyzed using TopScan v2.0 (CleverSys, Inc, Reston, VA, USA). Locomotor activity during these 60 min is reported in 5-min bins.
While the mice were separated into four experimental conditions for the evocation phase of the experiment, during the initial conditioning sessions there were only two treatment/experimental conditions (half mice receiving saline and half 10 mg/kg ketamine). Therefore, we reported open-field test results during the conditioning phase of our protocol as saline vs ketamine for both sexes separately.
Forced Swim Test (FST)
The FST is a common test to assess antidepressant-like efficacy of drugs in rodents, for which effects of ketamine have been noted both acutely (i.e. 1 hour), where the drug is still present at low levels in the brain of rodents, as well as following complete absence of the drug in the brain (i.e. 24 hours) to decrease immobility time (Autry et al. 2011; Li et al. 2010; Zanos et al. 2016). Mice underwent a 6-min swim session in clear Plexiglass cylinders (30 cm height × 20 cm diameter) filled to a 15 cm depth with tap water (23 ± 1 °C) performed in normal light conditions (800 Lux). Sessions were recorded using video cameras. Immobility time, defined as passive floating with no additional activity other than that necessary to keep the animal’s head above water, was scored from these videos for the last 4 min of the 6-min test by a trained observer blind to the treatment groups. Mice were tested in the FST 1 hour (immediately after OFT following the 7-week evocation) and re-tested 24 hours later for assessing ketamine-like long-lasting effects that may be evoked by the conditioning procedure.
Statistical Analysis
The conditioning parameters (reported in Table 1) and the OFT data were analyzed using a 2-way repeated measures ANOVA. Differences in FST immobility time between groups were assessed using a one-way ANOVA. ANOVAs were followed by Holm-Sidak multiple comparisons correction post-hoc test, when significance was reached (i.e., p<0.05). For comparison of the effect sizes of the placebo and ketamine groups (Sal/Ket and Ket/Sal compared with Sal/Sal), the Cohen’s d values were calculated using the following equation d = (|M1-M2| ) / (√Spooled), in which M = average of each group and Spooled = the pooled standard deviation of the two groups, i.e. (S12 + S22)/2. Following that, the d values were transformed to correlation coefficients using the formula r = d / √(d2 +4). For the z transformation the following formula was used z = 0.5 (ln (1 + r) - ln (1 − r)). To test whether the effect sizes were significantly different, the Zobserved between the groups of interest was calculated as Zobserved = (z1-z2) / (√(1 / (N1-3)) +(1 / (N2-3))), where N = animal number. The critical value for significance comparison of the Zobserved was set at 1.96 for an α = 0.05. Statistical outliers were determined and excluded from the dataset using the Grubb’s outlier test (Grubbs 1969). This resulted in three male mice being removed from the 24 hour FST (1 Ket/Sal, 1 Sal/Ket, and 1 Sal/Sal), and 1 female mouse from the 24 hour FST (Sal/Ket). One Sal-treated male mouse, as well as two Sal-treated and one Ket-treated female mice were excluded from the data set for total distance travelled and 60-minute center time activity due to a malfunction of the video capturing system towards the end of the recording. Male and female mice underwent behavioral testing on alternating weeks, and we therefore did not statistically compare the male and female results since they were tested at different times. Data were analyzed using GraphPad Prism software v6 and alpha was set at α = 0.05, with two-tailed comparisons.
Table 1:
Conditioning parameters
| Males | 2-way RM ANOVA | Females | 2-way RM ANOVA | |||
|---|---|---|---|---|---|---|
| Sal | Ket | SAL | Ket | |||
| Amount of chocolate consumed (mg) | ||||||
| Week 1 | 9.58 ± 2.79 | 14.58 ± 5.25 | Treatment: F[1, 46] = 0.21; p = 0.65 Time: F[2, 92] = 8.01; p < 0.001*** Interaction: F[2, 92] = 2.13; p = 0.13 |
43.75 ± 8.25 | 45.83 ± 10.60 | Treatment: F[1, 46] = 1.34; p = 0.25 Time: F[2, 92] = 15.27; p < 0.001*** Interaction: F[2, 92] = 1.77; p = 0.18 |
| Week 3 | 27.50 ± 6.95 | 26.25 ± 10.4 | 97.08 ± 11.25 | 72.50 ± 11.46 | ||
| Week 5 | 40.00 ± 9.38 | 24.17 ± 7.82 | 97.08 ± 15.76 | 71.67 ± 12.75 | ||
| Latency to first approach chocolate pellets (sec) | ||||||
| Week 1 | 25.17 ± 4.09 | 19.86 ± 3.26 | Treamentt: F[1, 46] = 0.16; p = 0.70 Time: F[2, 92] = 2.32; p = 0.10 Interaction: F[2, 92] = 0.08; p = 0.93 |
21.01 ± 2.49 | 18.00 ± 3.65 | Treatmetn: F[1, 46] = 2.61; p = 0.11 Time: F[2, 86] = 1.30; p = 0.28 Interaction: F[2, 86] = 1.13; p = 0.33 |
| Week 3 | 16.33 ± 3.24 | 17.73 ± 4.64 | 42.26 ± 18.57 | 13.88 ± 2.68 | ||
| Week 5 | 40.64 ± 13.32 | 35.17 ± 18.88 | 22.07 ± 10.85 | 9.48 ± 1.94 | ||
| Time spent in the center (first 5 min; sec) | ||||||
| Week 1 | 16.54 ± 1.84 | 15.96 ± 1.60 | Treatment: F[1, 46] = 0.42; p = 0.52 Time: F[2, 92] = 19.18; p < 0.001*** Interaction: F[2, 92] = 1.42; p = 0.25 |
17.16 ± 1.73 | 15.67 ± 1.68 | Treatment: F[1, 46] = 1.83; p = 0.18 Time: F[2, 92] = 3.70; p < 0.05* Interaction: F[2, 92] = 0.64; p = 0.53 |
| Week 3 | 12.06 ± 1.52 | 8.61 ± 1.36 | 15.64 ± 2.26 | 11.38 ± 1.57 | ||
| Week 5 | 7.99 ± 1.52 | 8.90 ± 1.50 | 14.87 ± 1.65 | 12.24 ± 1.65 | ||
| Time spent in the center (total 60 min; sec) | ||||||
| Week 1 | 252.7 ± 24.08 | 267.2 ± 26.21 | Treatment: F[1, 45] = 0.01; p = 0.91 Time: F[2, 90] = 1.04; p = 0.36 Interaction: F[2, 90] = 0.86; p = 0.43 |
284.9 ± 34.25 | 266.6 ± 20.55 | Treatment: F[1, 43] = 0.09; p = 0.77 Time: F[2, 86] = 2.38; p = 0.1 Interaction: F[2, 86] = 0.79; p = 0.24 |
| Week 3 | 244.8 ± 25.15 | 248.0 ± 18.32 | 321.1 ± 37.59 | 317.5 ± 23.08 | ||
| Week 5 | 289.4 ± 35.91 | 256.6 ± 31.29 | 292.9 ± 25.64 | 328.4 ± 29.18 | ||
Abbreviations: Ket, ketamine; RM, repeated measures; Sal, saline
RESULTS
Conditioning and evocation phases
Open-field locomotor activity
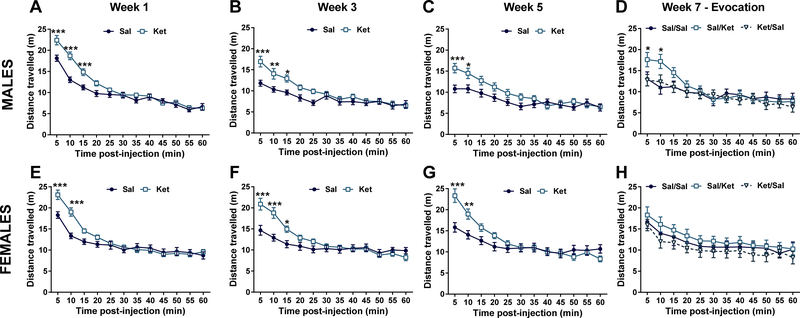
During each conditioning session we measured the locomotor activity of mice to assess for the expected N-methyl-D-aspartate receptor (NMDAR) inhibition induced hyperlocomotor actions of the drug (Irifune et al. 1995). For male mice, a significant effect of ketamine on locomotion during the OFT at conditioning week 1 (Treatment: F1,45 = 6.37, p < 0.05; Time: F11,495 = 139.0, p < 0.001; Interaction: F11,495 = 8.10, p < 0.001), week 3 (Treatment: F1,46 = 4.92, p < 0.05; Time: F11,506 = 36.12, p < 0.001; Interaction: F11,506 = 4.79, p < 0.001), and a statistical trend for treatment effect at week 5 (Treatment: F1,46 = 3.48, p = 0.07; Time: F11,506 = 33.21, p < 0.001; Interaction: F11,506 = 4.59, p < 0.001) was observed, indicating that ketamine effectively induced hyperlocomotor responses. Post-hoc comparisons revealed significant group differences during the first 5–15 minutes of the 60 minute OFT between ketamine- and saline-treated male mice (Figures 2A, B, and C). During the evocation phase in male mice, we did not observe a significant effect of experimental condition/treatment (F2,33= 0.53, p 0.59) but a significant time effect (F11,363 = 33.96, p < 0.001) and Drug x Time interaction: F22,363 = 3.58, p < 0.001). Post-hoc comparisons indicated that Sal/Ket mice displayed greater locomotion than did Ket/Sal and Sal/Sal group mice during the first 10 min following injection (Figure 2D).
Figure 2. Effects of ketamine and ketamine conditioning on locomotion.
In male mice ketamine resulted in increased open field locomotion immediately following administration for conditioning weeks 1, 3, and 5 (A, B, C). Ketamine administration during evocation in males increased locomotion relative to mice receiving saline, regardless of whether they received ketamine during conditioning (D). In female mice ketamine resulted in increased locomotion immediately following administration for conditioning weeks 1, 3, and 5 (E, F, G). No differences in locomotion were observed in female mice during the evocation phase (H). Sal=saline; Ket=ketamine 10 mg/kg, OFT=open-field test. Data are means ± S.E.M. *p<0.05, **p<0.01, ***p<0.001. N=24 per group in A, B, C, E, F, and G. N=12 per group in D and H.
For female mice, ANOVAs indicated a significant interaction between treatment (ketamine vs saline) and time at conditioning week 1 (Treatment: F1,43 = 2.16 p = 0.15; Time: F11,473 = 101.60, p < 0.001; Interaction: F11,473 = 9.56, p < 0.001), week 3 (Treatment: F1,46 = 2.78, p = 0.10; Time: F11,506 = 44.08, p < 0.001; Interaction: F11,506 = 9.81, p < 0.001), and week 5 (Treatment: F1,46 = 1.21, p = 0.28; Time: F11,506 = 61.63, p < 0.001; Interaction: F11,506 = 12.43, p < 0.001). Post-hoc comparisons identified ketamine-induced increases in locomotion typically in the first 5–15 minutes of the OFT between the ketamine- vs saline-treated mice during each conditioning exposure (Figures 2E, F, and G). During the evocation phase, we did not observe a significant effect of experimental condition/treatment or Condition × Time interaction in female mice (Condition: F2,33 = 0.93, p = 0.41; Interaction: F22,363 = 0.31, p = 0.99; Figure 2H), but a significant effect of time (Time: F11,363 = 19.69, p < 0.001; Figure 2H).
Conditioning parameters
There was no significant effect of ketamine on the amount of chocolate consumed, latency to first approach chocolate pellets, and time spent in the center during the first 5 min and total 60 min of each open field exposure (Table 1). We cannot exclude sex differences in these measurements as well as effectiveness of the conditioning procedure itself because mice were not stratified by sex before assignment to each group.
Conditioned effects in the forced-swim test
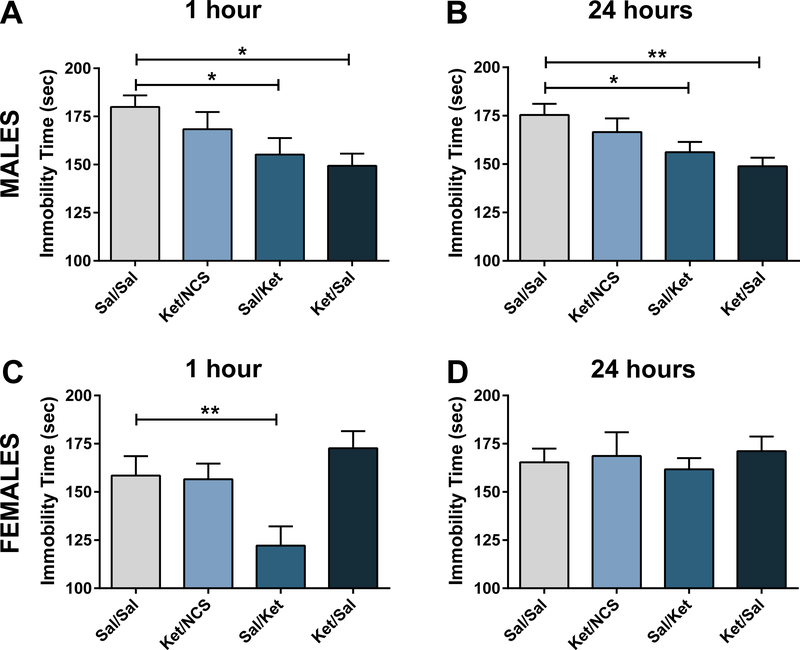
For the male mice, one-way ANOVA analysis revealed a significant effect of treatment/group in the FST (F3,44 = 3.28, p < 0.05). Post-hoc comparisons revealed that mice conditioned with saline injections that received an acute ketamine injection during evocation period (i.e., Sal/Ket group), and “placebo” mice that were conditioned with ketamine and received saline injection during the evocation period (i.e., Ket/Sal group) manifested significantly less immobility time than the Sal/Sal control group (Figure 3A). The effect size for the antidepressant-like effects in the Sal/Ket and Ket/Sal was comparable as indicated by the Cohens’d value comparisons (Sal/Ket: Cohen’s d = 0.97 vs Ket/Sal: 1.44, effect sizes: r = 0.44 vs 0.58) that was not significant (Fisher r-to-z transformation test: z = 0.47 vs 0.67, z observed= 0.43; critical significance value: 1.96 for α= 0.05), suggesting that the CS elicited a ketamine-like effect comparable to the effect elicited by a single ketamine administration.
Figure 3. Effects of ketamine and ketamine conditioning in the forced swim test.
In male mice, condition stimulus (CS) exposure without recent ketamine administration (Ket/Sal) reduced immobility 1 hour (A) and 24 hours (B) after the exposure to the conditioning environment. In female mice, CS exposure without a further ketamine administration had no effect 1 hour (C) or 24 hours (D) after exposure to the conditioning environment. Sal=Saline; Ket=ketamine; NCS=No Conditioning Stimulus. Data are means ± S.E.M. *p<0.05, **p<0.01, ***p<0.001. N=11–12 per group.
ANOVA analysis also revealed significant effect of treatment at the 24-hour time point following injection in male mice (F3,41 = 3.98, p < 0.05). Similar to the 1-hour post-injection findings, post-hoc comparisons revealed that Sal/Ket and Ket/Sal male mice manifested significantly less immobility time compared with their respective Sal/Sal controls 24 hours following evocation (Figure 3B) indicating that the CS induced sustained ketamine-like effects. The control (Ket/NCS) mice that received ketamine during conditioning and had no exposure to the conditioning stimulus during evocation did not manifest decreased immobility time compared to Sal/Sal controls neither at 1- or 24-hours following evocation (Figures 3A, B). The effect size for the antidepressant-like effects in the Sal/Ket and Ket/Sal was comparable as indicated by the Cohens’d value comparisons (Sal/Ket: Cohen’s d = 1.05 vs Ket/Sal: 1.56, effect sizes: r = 0.46 vs 0.62) that was not significant (Fisher r-to-z transformation test: z = 0.50 vs 0.0.72, z observed= 0.46; critical significance value: 1.96 for α = 0.05).
For the female mice, one-way ANOVA analysis revealed significant differences between groups in immobility time one hour following injection (F3,44 = 5.25, p <0.01). In contrast to the male mice described above, post-hoc comparisons revealed that only the female mice that received an acute ketamine injection during the evocation period and which had been previously conditioned using saline injections (i.e., Sal/Ket group) manifested lower immobility time compared with the control Sal/Sal mice (Figure 3C). We did not observe any significant difference between groups 24 hours after injection in female mice (F3,43 = 0.22, p = 0.88) (Figure 3D).
DISCUSSION
Using ketamine, we established a classical conditioning model of placebo antidepressant-like effects in mice. Specifically, we showed that following three pairings of ketamine administration with a CS, the subsequent exposure to the CS can significantly reduce immobility time in the forced swim test both 1 hour and 24 hours after exposure. Our results were sex-dependent, since only males manifested a conditioned antidepressant-like response. Presentation of the CS did not produce increased locomotion in the open-field test, indicating that the psychomotor effects of ketamine were not evoked by the same mechanisms as the placebo antidepressant-relevant effects. These results validate a platform for future research on the neurobiological mechanisms of placebo antidepressant effects.
Work over the last two decades has greatly expanded our knowledge on placebo effects, and the mechanisms underlying these effects are currently an active area of investigation (Colagiuri et al. 2015; Colloca 2019; Colloca et al. 2013; Peiris et al. 2018). The classical conditioning model employed in the present study has previously been used in human placebo research on pain (Amanzio and Benedetti 1999; Montgomery and Kirsch 1996; Pollo et al. 2001), Parkinson’s disease (Benedetti et al. 2004), and immunosuppression (Goebel et al. 2002). Pre-clinically, most research designed to study placebo effects have utilized a classical conditioning model initially used in pain research (Keller et al. 2018). This model has since allowed for mechanistic evaluation of general placebo mechanisms, including identification of specific molecular pathways involved in the emergence of placebo analgesic effects (Guo et al. 2010; Guo et al. 2011; Zhang et al. 2013). However, there is no prior research to understand the nature and the mechanisms underlying antidepressant placebo effects, which commonly occur in clinical studies of depression treatment (Furukawa et al. 2016; Keller et al. 2018; Kirsch 2010; 2014). The lack of previous research in this area is mainly due to the fact that classical conditioning requires brief temporal delays between the stimulus and the response, whereas typical antidepressants (i.e. monoamine-based antidepressants) have a slow therapeutic onset of weeks to months, making a classical conditioning model with predictive validity challenging. In contrast to the slow onset of therapeutic actions of classical antidepressants, ketamine exerts rapid antidepressant actions both in humans (Berman et al. 2000; Fava et al. In press; Lapidus et al. 2014; Murrough et al. 2013; Singh et al. 2016; Zarate et al. 2006), and rodents (Autry et al. 2011; Li et al. 2010; Ramaker and Dulawa 2017; Zanos and Gould 2018).
Here, we showed that ketamine-like placebo behavioral effects can be observed in preclinical tests and can be long-lasting (i.e., 24 hours post-CS exposure). Although these findings clearly show the feasibility of inducing placebo antidepressant-relevant effects in male mice, a limitation of the present study is that we used the FST, which is a test sensitive to acute administration of classical antidepressants (Petit-Demouliere et al. 2005). However, ketamine has the unique action, compared to such classical antidepressants, to decrease immobility time in the FST even after 24 hours post-injection (Maeng et al. 2008; Zanos et al. 2016; Zanos et al. 2015), a time point where no drug levels are present in the brain of rodents (Zanos et al. 2016). Notably, we make no argument that the beneficial effects of ketamine observed clinically (with either a single or repeated dosing) or pre-clinically are simply a placebo effect. While true blinding of participants in clinical trials assessing ketamine’s effects is difficult due to the robust psychosomatic symptoms of ketamine at the antidepressant doses given in patients (Berman et al. 2000; Krystal et al. 1994), ketamine is repeatedly more effective compared with placebo in the clinical studies published to date (see Berman et al. 2000; Fava et al. In press; Lapidus et al. 2014; Singh et al. 2016; Zarate et al. 2006). The antidepressant effects of ketamine has also been confirmed when ketamine is compared to a psychoactive placebo such as a benzodiazepine (Murrough et al. 2013), and by meta-analyses of available studies (Fond et al. 2014). Additionally, preclinical work in rodents indicates that even without any previous drug exposure (therefore no learned responses about the impact of the drug are acquired), acute ketamine administration exerts behavioral effects in multiple clinically antidepressant-relevant behavioral assays (Autry et al. 2011; Li et al. 2010; Ramaker and Dulawa 2017; Zanos and Gould 2018; Zanos et al. 2016). Our finding that ketamine-like behavioral effects can be intentionally induced using pre-exposure to effective ketamine paired with CS exposure (i.e. placebos) may have translational relevance. Dose-extending placebos are used in pain and placebo research (Colloca et al. 2016a), and could have applicability for the treatment of depression.
While male mice manifested ketamine-like placebo effects, we did not observe effects of antidepressant conditioning in female mice. Also, we note that the 10 mg/kg dose of ketamine (in the absence of CS) was effective in males, but not effective in females in inducing antidepressant-like effects 24 h after administration. This cannot be explained through lack of efficacy of ketamine in females, as we also show that a single injection of ketamine in females significantly reduced immobility time relative to a saline injection at the 1 h time point. Substantial research has indicated effects of sex in the antidepressant-relevant actions of ketamine in rodents. Specifically, more potent antidepressant behavioral responses of a single administration of ketamine have typically been observed in female compared to male rats and mice acutely and/or 24 hours post-injection (Carrier and Kabbaj 2013; Sarkar and Kabbaj 2016; Zanos et al. 2016). Thus, a limitation of the present study was that only a single dose of ketamine was utilized. It is possible that the most effective dose in females in exerting effects at 24 hours, as well as placebo conditioning effects, differs from the most effective dose in males. Additionally, it may be that conditioning procedures may vary between male and female mice. Though not assessed here, a different CS may allow for conditioning of antidepressant responses in females. Moreover, we cannot exclude sex differences in the effectiveness of the conditioning procedure itself, which would in turn impact placebo effects. We also did not control for female estrous cycle phase, which could have interacted with conditioned responses. However, we note that there was not greater variability in the female, compared to the male group data, as would be expected if activational effects of gonadal hormones influenced results. Future experiments are needed to assess whether the effects observed, including sex differences, are due to the specific CS utilized, the strain of mice used and/or other environmental factors.
There is limited research on sex effects in placebo behavioral responses in either clinical or preclinical research. Several studies have observed sex differences in placebo effects in humans (see Vambheim and Flaten 2017 meta-analysis). In some studies, males manifested a greater response to placebos than females (Abrams and Kushner 2004; Aletky and Carlin 1975; Aslaksen et al. 2011; Aslaksen and Flaten 2008; Bjorkedal and Flaten 2011); but there are also opposite results (Colloca et al. 2016b; Haltia et al. 2008; Klosterhalfen et al. 2009; Krummenacher et al. 2014) suggesting that several factors may play a role in dimorphic responses to placebos. There is some evidence that these sex differences might be attributed to differences in stress reactivity between males and females (Aslaksen et al. 2011), but the exact mechanisms remain still unclear. Unfortunately, previous classical conditioning preclinical models for the study of placebo effects have primarily used male animals (Hadamitzky et al. 2018; Keller et al. 2018), making it difficult to completely understand the nature and generalizability of the sex-dependent effects observed in the present study. However, we note that one previous study failed to condition placebo analgesia in female rats (McNabb et al. 2014). Continued research is necessary to understand the mechanisms of sex differences in placebo effects.
Our work demonstrates, for the first time to our knowledge, the feasibility of producing antidepressant-like placebo effects in mice, using a classical conditioning model. Herein, we used a CS (placebo procedure) during the acquisition phase that included rodent handling, the saline vs ketamine i.p. injection, and an immediate 60-min exposure to the open-field arena containing ~0.5g sugar-free chocolate and blue light conditions. While this procedure was effective in eliciting a conditioned response in males, our experiments did not discern which aspects of the procedure are necessary or sufficient to exert the conditioned effect. We note that pioneering studies of conditioned placebo effects have used even simpler CSs (Herrnstein 1962). Future research can further investigate the requirements for successful CRs and placebo effects, and the mechanisms and circumstances under which sex differences play a role.
These results establish a protocol for future investigation of the neurobiological mechanisms of placebo-induced antidepressant effects and possible sex differences in these outcomes, which is critically needed. Since placebo effects are believed to operate, at least in part, by mechanisms independent of the compound’s direct pharmacodynamic effects (Benedetti et al. 2005; Colloca 2019; Mayberg et al. 2002; Stein and Mayberg 2005), the rodent model of antidepressant-like placebo effects in male mice described in the present study might offer a novel avenue for investigation of circuits that can more effectively treat depression and for further defining ketamine’s mechanism of antidepressant action.
ACKNOWLEDGMENTS
This research was supported by NIH/NIMH R01-MH107615 and VA Merit Award 1I01BX004062 to TDG. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
DISCLOSURES
TDG has received research funding from Allergan, and Roche Pharmaceuticals, and consultant fees from FSV7 LLC during the preceding three years. LC reported having received support for Invited Lectures outside the submitted work.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Abrams K, Kushner MG (2004) The moderating effects of tension-reduction alcohol outcome expectancies on placebo responding in individuals with social phobia. Addict Behav 29: 1221–4. [DOI] [PubMed] [Google Scholar]
- Aletky PJ, Carlin AS (1975) Sex differences and placebo effects: motivation as an intervening variable. J Consult Clin Psychol 43: 278. [DOI] [PubMed] [Google Scholar]
- Amanzio M, Benedetti F (1999) Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 19: 484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G (2001) Placebo response in depression: bane of research, boon to therapy. Br J Psychiatry 178: 192–4. [DOI] [PubMed] [Google Scholar]
- Aslaksen PM, Bystad M, Vambheim SM, Flaten MA (2011) Gender differences in placebo analgesia: event-related potentials and emotional modulation. Psychosomatic medicine 73: 193–9. [DOI] [PubMed] [Google Scholar]
- Aslaksen PM, Flaten MA (2008) The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosomatic medicine 70: 811–8. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L (2004) Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci 7: 587–8. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK (2005) Neurobiological mechanisms of the placebo effect. J Neurosci 25: 10390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biological psychiatry 47: 351–4. [DOI] [PubMed] [Google Scholar]
- Bjorkedal E, Flaten MA (2011) Interaction between expectancies and drug effects: an experimental investigation of placebo analgesia with caffeine as an active placebo. Psychopharmacology (Berl) 215: 537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70: 27–34. [DOI] [PubMed] [Google Scholar]
- Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, Colloca L (2015) The placebo effect: From concepts to genes. Neuroscience 307: 171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L (2019) The Placebo Effect in Pain Therapies. Annu Rev Pharmacol Toxicol 59: 191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Enck P, DeGrazia D (2016a) Relieving pain using dose-extending placebos: a scoping review. Pain 157: 1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Klinger R, Flor H, Bingel U (2013) Placebo analgesia: psychological and neurobiological mechanisms. Pain 154: 511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Pine DS, Ernst M, Miller FG, Grillon C (2016b) Vasopressin Boosts Placebo Analgesic Effects in Women: A Randomized Trial. Biological psychiatry 79: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI (In press) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry: doi: 10.1038/s41380-018-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L (2014) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 231: 3663–76. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J (2010) Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Cipriani A, Atkinson LZ, Leucht S, Ogawa Y, Takeshima N, Hayasaka Y, Chaimani A, Salanti G (2016) Placebo response rates in antidepressant trials: a systematic review of published and unpublished double-blind randomised controlled studies. Lancet Psychiatry 3: 1059–1066. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, Canbay AE, Michel MC, Heemann U, Schedlowski M (2002) Behavioral conditioning of immunosuppression is possible in humans. FASEB J 16: 1869–73. [DOI] [PubMed] [Google Scholar]
- Grubbs FE (1969) Procedures for Detecting Outlying Observations in Samples. Technometrics 11: 1–21. [Google Scholar]
- Guo JY, Wang JY, Luo F (2010) Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. J Psychopharmacol 24: 1561–7. [DOI] [PubMed] [Google Scholar]
- Guo JY, Yuan XY, Sui F, Zhang WC, Wang JY, Luo F, Luo J (2011) Placebo analgesia affects the behavioral despair tests and hormonal secretions in mice. Psychopharmacology (Berl) 217: 83–90. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Sondermann W, Benson S, Schedlowski M (2018) Placebo Effects in the Immune System. Int Rev Neurobiol 138: 39–59. [DOI] [PubMed] [Google Scholar]
- Haltia LT, Rinne JO, Helin S, Parkkola R, Nagren K, Kaasinen V (2008) Effects of intravenous placebo with glucose expectation on human basal ganglia dopaminergic function. Synapse 62: 682–8. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ (1962) Placebo effect in the rat. Science 138: 677–8. [DOI] [PubMed] [Google Scholar]
- Hoflich A, Ganger S, Tik M, Hahn A, Kranz GS, Vanicek T, Spies M, Kraus C, Windischberger C, Kasper S, Winkler D, Lanzenberger R (2017) Imaging the neuroplastic effects of ketamine with VBM and the necessity of placebo control. Neuroimage 147: 198–203. [DOI] [PubMed] [Google Scholar]
- Irifune M, Shimizu T, Nomoto M, Fukuda T (1995) Involvement of N-methyl-D-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice. Pharmacol Biochem Behav 51: 291–6. [DOI] [PubMed] [Google Scholar]
- Keller A, Akintola T, Colloca L (2018) Placebo Analgesia in Rodents: Current and Future Research. Int Rev Neurobiol 138: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I (2010) Review: benefits of antidepressants over placebo limited except in very severe depression. Evid Based Ment Health 13: 49. [DOI] [PubMed] [Google Scholar]
- Kirsch I (2014) The emperor’s new drugs: medication and placebo in the treatment of depression. Handb Exp Pharmacol 225: 291–303. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (2008) Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 5: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Sapirstein G (1998) Listening to Prozac but hearing placebo: A meta-analysis of antidepressant medication. Prevention & Treatment 1: No Pagination Specified-No Pagination Specified. [Google Scholar]
- Klosterhalfen S, Kellermann S, Braun S, Kowalski A, Schrauth M, Zipfel S, Enck P (2009) Gender and the nocebo response following conditioning and expectancy. J Psychosom Res 66: 323–8. [DOI] [PubMed] [Google Scholar]
- Krummenacher P, Kossowsky J, Schwarz C, Brugger P, Kelley JM, Meyer A, Gaab J (2014) Expectancy-induced placebo analgesia in children and the role of magical thinking. J Pain 15: 1282–93. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of general psychiatry 51: 199–214. [DOI] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biological psychiatry 76: 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological psychiatry 63: 349–52. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA (2002) The functional neuroanatomy of the placebo effect. Am J Psychiatry 159: 728–37. [DOI] [PubMed] [Google Scholar]
- McNabb CT, White MM, Harris AL, Fuchs PN (2014) The elusive rat model of conditioned placebo analgesia. Pain 155: 2022–32. [DOI] [PubMed] [Google Scholar]
- Montgomery G, Kirsch I (1996) Mechanisms of Placebo Pain Reduction: An Empirical Investigation. Psychological Science 7: 174–176. [Google Scholar]
- Murrough JW, losifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170: 1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris N, Blasini M, Wright T, Colloca L (2018) The Placebo Phenomenon: A Narrow Focus on Psychological Models. Perspect Biol Med 61: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M (2005) Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 177: 245–55. [DOI] [PubMed] [Google Scholar]
- Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F (2001) Response expectancies in placebo analgesia and their clinical relevance. Pain 93: 77–84. [DOI] [PubMed] [Google Scholar]
- Ramaker MJ, Dulawa SC (2017) Identifying fast-onset antidepressants using rodent models. Molecular Psychiatry 22: 656. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–17. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M (2016) Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biological psychiatry 80: 448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016) A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry 173: 816–26. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Mayberg H (2005) Placebo: the best pill of all. CNS Spectr 10: 440–2. [DOI] [PubMed] [Google Scholar]
- Vambheim SM, Flaten MA (2017) A systematic review of sex differences in the placebo and the nocebo effect. J Pain Res 10: 1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Farmer C, Ballard ED, Mathew SJ, Grunebaum MF, Murrough JW, Sos P, Wang G, Gueorguieva R, Zarate CA (2019) Impact of midazolam vs. saline on effect size estimates in controlled trials of ketamine as a rapid-acting antidepressant. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533: 481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Piantadosi SC, Wu HQ, Pribut HJ, Dell MJ, Can A, Snodgrass HR, Zarate CA Jr., Schwarcz R, Gould TD (2015) The Prodrug 4-Chlorokynurenine Causes Ketamine-Like Antidepressant Effects, but Not Side Effects, by NMDA/GlycineB-Site Inhibition. The Journal of pharmacology and experimental therapeutics 355: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry 63: 856–64. [DOI] [PubMed] [Google Scholar]
- Zhang RR, Zhang WC, Wang JY, Guo JY (2013) The opioid placebo analgesia is mediated exclusively through mu-opioid receptor in rat. Int J Neuropsychopharmacol 16: 849–56. [DOI] [PubMed] [Google Scholar]