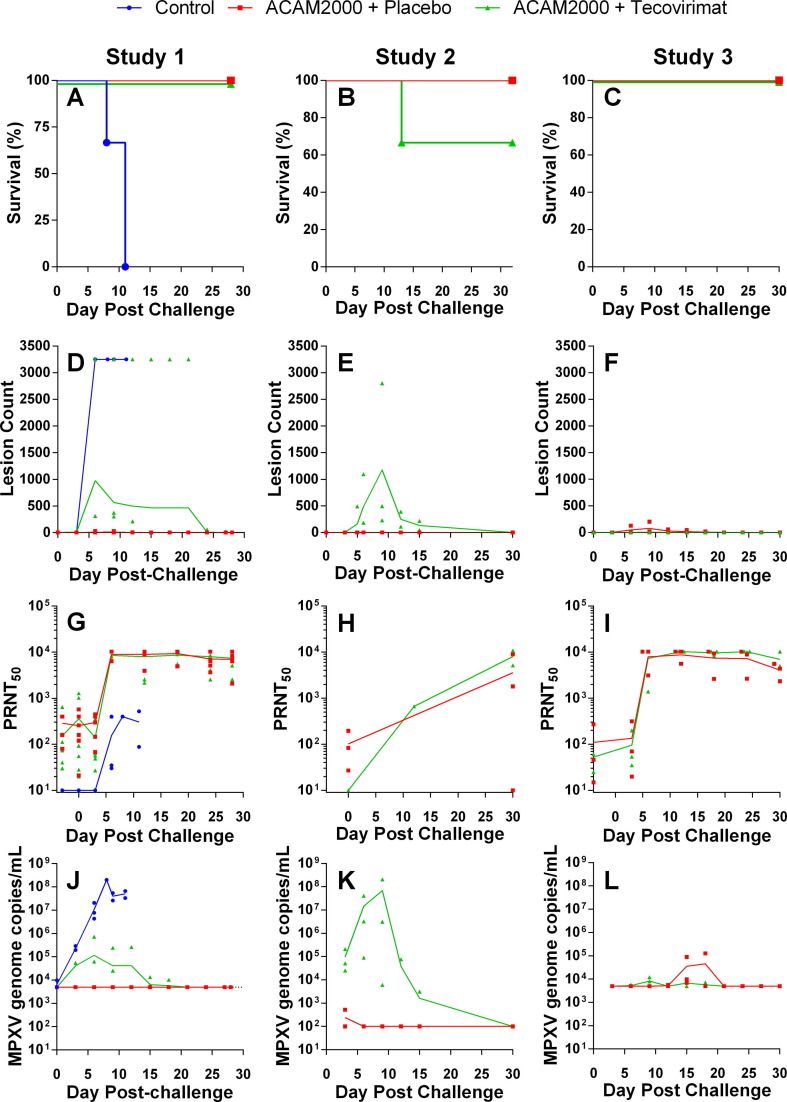
Fig. 3.
Protective efficacy of ACAM2000 given in combination with tecovirimat in cynomolgus (Studies 1 and 2) and rhesus (Study 3) macaques. NHPs were inoculated by epidermal scarification with ACAM2000 or vaccine diluent and concurrently treated with placebo or tecovirimat (10 mg/kg, once daily) for 14 days. NHPs were challenged with a lethal dose of MPXV, Zaire 79 via the intravenous route 45 (Study 1), 30 (Study 2) or 32 (Study 3) days following vaccination with ACAM200 or vaccine diluent. Following MPXV challenge, NHPs were monitored for survival (A–C), group mean total body lesion counts (D–F), PRNT50 (G–I), and MPXV genome copies in blood (J–L). LLOQ for MPXV genome copies/mL in Studies 1 and 3 (Panels J and K respectively) was 5 × 103. Study 2 (Panel K) LLOQ was 1 × 102. Study Group sizes were as follows: Study 1 Unvaccinated + placebo Control (n = 3), ACAM2000 + Placebo (n = 6) and ACAM2000 + tecovirimat (n = 7); Study 2 ACAM2000 + Placebo (n = 3) and ACAM2000 + tecovirimat (n = 3); Study 3 ACAM2000 + Placebo (n = 3) and ACAM2000 + tecovirimat (n = 3). Study Group sizes were as described in Fig. 2, with the exception that the Study 1 ACAM2000 + Placebo group size is reduced by one from the pre-challenge group size due to censoring of one NHP prior to MPXV challenge due to health issues unrelated to study procedures. Individual animal data are plotted on all figures. Connecting lines are plotted through group mean values for lesion counts and group geometric mean values for PRNT50 and MPXV genome measurements.