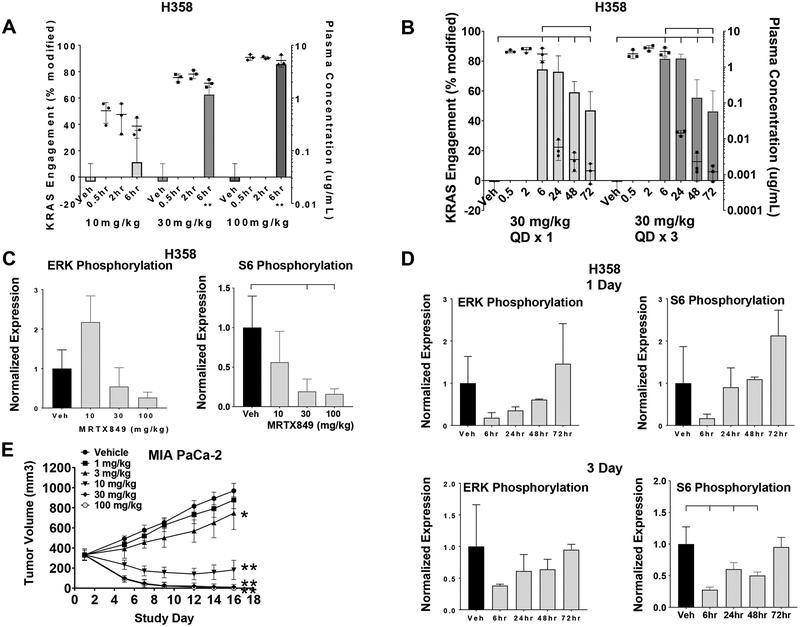
Figure 2.
MRTX849 modifies KRASG12C and inhibits KRAS signaling and tumor growth in vivo.
(A) MRTX849 was administered orally as a single dose to mice bearing established H358 xenografts (average tumor volume ~350 mm3) at 10, 30 and 100 mg/kg. KRAS modification and MRTX849 plasma concentration data from n=3 mice are shown as mean +/− standard deviation (SD). KRASG12C modification was statistically significant vs vehicle control using the two-tailed Student’s t-test. “**” indicates p-value < 0.01.
(B) MRTX849 was administered orally as a single dose or daily for three days to mice bearing established H358 xenografts (average tumor volume ~350 mm3) at 30 mg/kg. Plasma was collected 0.5, 2, 6, 24, 48 and 72 hours post administration of the last dose and tumors were collected 6, 24, 48 and 72 hours post dose. KRASG12C modification and MRTX849 plasma concentration data are shown from n=3 mice as mean +/− SD. Induction of modified KRASG12C protein at all time points was determined to be statistically significant vs. vehicle control using two-way ANOVA. In addition, induction of modified KRASG12C protein at 72 hours in Day 1 samples and 48 and 72 hours in Day 3 samples was statistically significant vs the 6-hour time point. Brackets indicate p-value < 0.05 as compared from left-most sample.
(C) MRTX849 was administered as in (A). Tumors were collected six hours post dose and total and phosphorylated ERK1/2 and total and phosphorylated S6 were analyzed by immunoblot and quantified by densitometric analysis. Relative fluorescent intensity of pERK1/2 and pS6 were normalized by dividing pERK1/2 and pS6 by total ERK1/2 and total S6, respectively. Vehicle tumors were normalized to 1 by dividing all average values by the vehicle value. Average pERK1/2 and pS6 values were divided by the average value in vehicle-treated tumors. Data shown represent the average of 2–3 tumors per treatment group plus SD. Reduction of pS6 relative fluorescent intensity was determined to be statistically significant vs vehicle control using the two-tailed Student’s t-test. Brackets indicate p-value <0.05 compared to left-most sample.
(D) MRTX849 was administered as in (B). Tumors were collected 6, 24, 48 or 72 hours post administration of the last dose and total and phosphorylated ERK1/2 and total and phosphorylated S6 were analyzed as in (C). Data shown represent the average of 3–4 tumors per treatment group plus SD. Reduction of pS6 relative fluorescent intensity on Day 3 was determined to be statistically significant vs vehicle control using two-way ANOVA. Brackets indicate p-value < 0.05 compared to left-most sample.
(E) MRTX849 was administered via daily oral gavage at the doses indicated to mice bearing established MIA PaCa-2 xenografts. Dosing was initiated when tumors were ~350 – 400 mm3. MRTX849 was administered to mice daily until Day 16. Data are shown as mean tumor volume +/− standard error of the mean (SEM). Tumor volumes at Day 16 were determined to be statistically significant vs vehicle control two-tailed Student’s t-test. “**” indicates p-value < 0.01. “*” indicates p-value < 0.05.