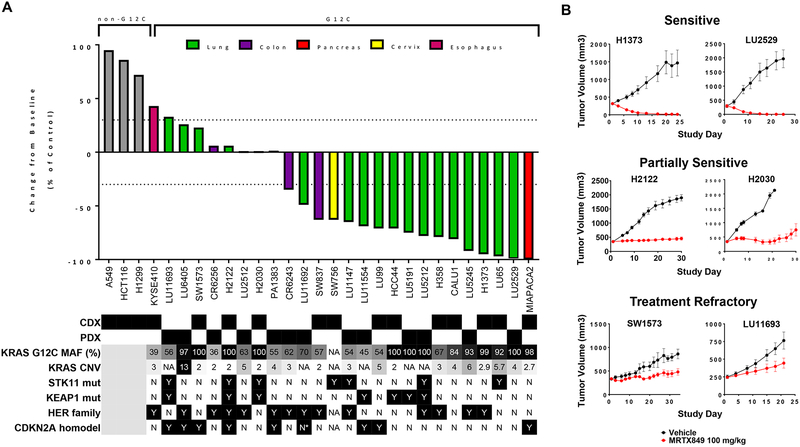
Figure 3.
Anti-tumor activity of MRTX849 in KRASG12C-mutant and non KRASG12C-mutant human tumor xenografts models.
(A) MRTX849 was administered via oral gavage at 100 mg/kg QD to mice bearing the cell line xenograft or PDX model indicated. Dosing was initiated when tumors were, on average, ~250 – 400 mm3. MRTX849 was formulated as a free base and resuspended as a solution in 10% Captisol, 50 mM citrate buffer, pH 5.0. The % change from baseline control was calculated at Day 19–22 for most models. Statistical significance was determined for each model and is shown in Table S6. Status of mutations and alterations in key genes are shown below each model. MAF (%) - Percent KRASG12C-mutant allele fraction by RNAseq; CNV – Copy number variation; * denotes very high CDK4 expression by RNAseq and possible amplification. HER family status was determined by averaging EGFR, ERBB2 and ERBB3 RNAseq expression for CDX (CCLE) or PDX (Crown huBase) models. Positive HER family calls denote greater than the median expression of the models tested. CDX and PDX model HER family calls were determined independently.
(B) Tumor growth inhibition plots from representative xenograft models that were categorized as sensitive, partially sensitive and treatment refractory.