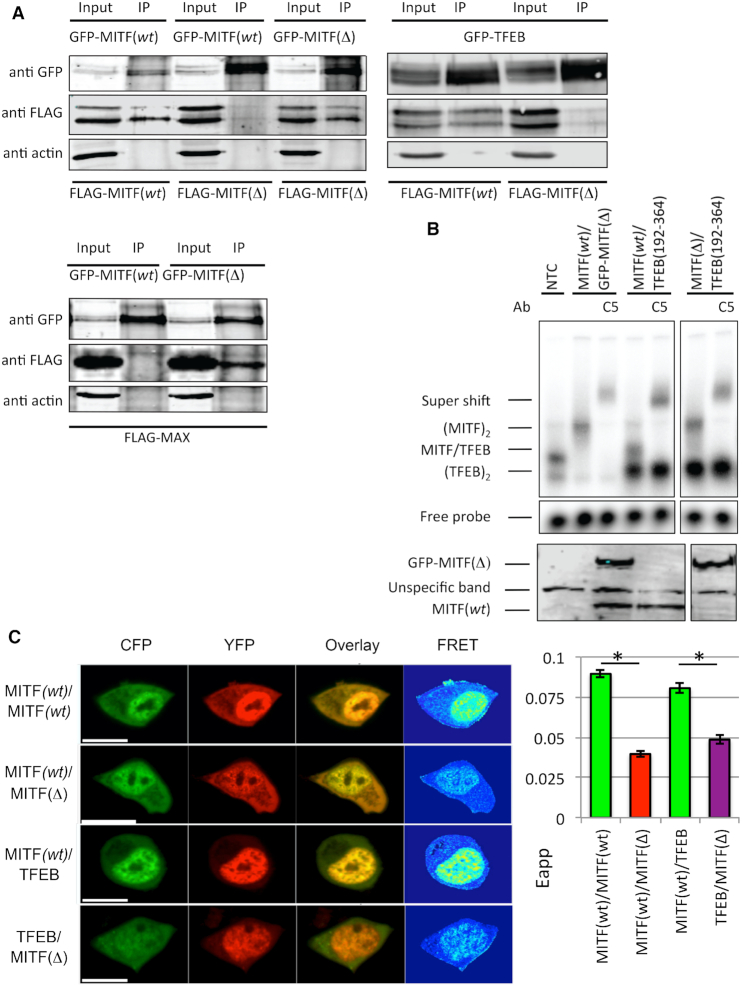
Figure 5.
The effects of stammer removal on the ability of MITF to heterodimerize with other bHLH-ZIP transcription factors. (A) FLAG-tagged MITF(wt), MITF(Δ) and MAX were expressed together with either GFP-tagged MITF(wt), MITF(Δ) and TFEB in 501 Mel cells. Protein interactions were analyzed by co-immunoprecipitation (co-IP) by Western blot detection of either anti-FLAG or anti-GFP antibodies. Negative control (transfected EV-GFP) is shown in Supplementary Figure S4. (B) Upper panel, EMSA showing the binding of MITF(wt), MITF(Δ) and TFEB to the M-box recognition element. Supershifts with a MITF-specific antibody are indicated, confirming the specificity of the gel shifts. In the presence of M-box MITF(wt)/TFEB heterodimers were observed but no MITF(Δ)/TFEB heterodimers. Lower panel, Western blot confirming in vitro translation products of the GFP-tagged MITF(Δ) (top panel) and MITF(wt) (bottom panel) constructs. (C) FRET dimerization experiments in HEK 293 cells. Left panel, representative images of FRET experiments of MITF(wt)2, MITF(wt)/MITF(Δ), MITF(wt)/TFEB and MITF(Δ)/TFEB labeled with CFP and YFP, respectively. The lengths of scale bars correspond to 10 μm. Right panel, quantification of the data. Error bars are defined as standard error of the mean (SEM). Asterisks (*) indicate a significant difference (P < 0.05) of FRET values. Statistics are derived from student's t‐test distribution.