Abstract
Background
The objective was to evaluate the risk and predictors of developing leptomeningeal disease (LMD) in patients with brain metastases treated with 5-fraction hypofractionated stereotactic radiotherapy (HSRT).
Methods
Patients treated with HSRT for intact brain metastases and/or surgical cavities were reviewed from a prospectively maintained database. Radiographic patterns of LMD were classified as focal classical, diffuse classical, focal nodular, and diffuse nodular.
Results
HSRT was delivered, most commonly 30 Gy in 5 fractions, to 320 intracranial lesions (57% intact and 43% surgical cavities) in 235 patients. The median follow-up was 13.4 months (range, 0.8 to 60 mo). LMD developed in 19% of patients with a 1-year LMD rate of 12%. From the diagnosis of LMD, the median overall survival (OS) was 3.8 months (range, 2–20.8 mo). The most common LMD pattern was diffuse nodular (44%). No difference in OS was observed between LMD patterns (P = 0.203). Multivariable analysis identified surgical cavities at significantly higher risk of LMD compared with intact lesions (odds ratio [OR] = 2.30, 95% CI: 1.24, 4.29, P = 0.008). For cavities, radiosensitive tumors (OR = 2.35, 95% CI: 1.04, 5.35, P = 0.041) predicted for LMD, while, for intact metastases, patients receiving treatment with targeted agents or immunotherapy (TA/I) were at lower risk (OR = 0.178, 95% CI: 0.04, 0.79, P = 0.023).
Conclusions
Patients who had a brain metastasis resected were at an increased risk of LMD. OS was poor despite treatment of LMD, and no differences in OS based on the pattern of LMD was observed. Treatment with TA/I was observed to be protective against LMD and requires further study.
Keywords: brain metastases, hypofractionated, leptomeningeal, radiosurgery, radiotherapy
Key Points.
1. HSRT for resected brain metastases is at greater risk of LMD than intact metastases.
2. Targeted agents/immunotherapy use was associated with decreased LMD risk.
3. No differences in survival were observed between radiographic patterns of LMD.
Importance of the Study.
A cohort of 112 patients with intact metastases and 123 patients with surgical cavities treated with HSRT demonstrated 1-year LMD rates of 6% and 20%, respectively. From the date of LMD, survival was poor, with a median survival of 3.8 months and 6- and 12-month OS rates of 42% and 15%, respectively. Following treatment with WBRT, the median survival was 5.3 months (95% CI: 3.2, 8 mo) compared with 1.6 months (95% CI: 1.1, 8.9 mo) with no further treatment (P = 0.111). No differences in OS were observed between radiographic LMD patterns (focal nodular vs diffuse nodular vs focal classic vs diffuse classic; and nodular vs classic). Surgical cavities were at greater risk of developing LMD (OR = 2.30, P = 0.008). For intact metastases, treatment with TA/I reduced the risk of LMD (OR = 0.178, P = 0.023).
Leptomeningeal disease (LMD) is a pattern of neoplastic disease progression characterized by the dissemination of neoplastic cells throughout the leptomeninges and/or cerebrospinal fluid.1 The most common intracranial sites of LMD include the cerebellum, occipital lobe, cranial nerves VII/VIII, and ependymal lining of the lateral ventricles.2 Several groups have acknowledged the existence of distinct radiographic patterns of LMD,3–5 including descriptions such as loculated, nodular, linear, curvilinear, non-adherent, and classic.3,5,6 Recently, there has been renewed interest in describing risk factors associated with developing LMD in patients with brain metastases, as well as outcomes based on an accepted classification scheme.7
Our institutional practice has been to deliver hypofractionated stereotactic radiotherapy (HSRT), as opposed to single-fraction stereotactic radiosurgery (SF-SRS), to surgical cavities and larger intact brain metastases with the goal of safely optimizing local control (LC). This shift in practice has been justified based on studies suggesting that SF-SRS results in inferior LC rates for larger metastases and increased rates of radiation necrosis. For example, Minnitti et al reported that brain metastases >2 cm have a 1-year LC rate of 91% following HSRT versus 77% with SF-SRS.8 Similar results have been observed for surgical cavities, where HSRT can achieve 1-year LC rates of 84–93% with reasonable 1-year radiation necrosis rates of 4.2–7%.9,10 The suboptimal LC rates observed with SF-SRS for resected and larger intact metastases may be explained by the dose de-escalation that occurs with increasing target size, which is required to mitigate the risk of radiation necrosis.11 Specific to surgical cavities, it is also suggested that inadequate target coverage may contribute to the poor rates of LC observed with SF-SRS alone in the randomized trials that range from 61% to 72%.12,13
We hypothesized that HSRT may influence LMD rates given the inherent dose escalation to surgical cavities and intact metastases. Furthermore, for the cavity cohort we postulated that the addition of a generous margin along the meninges in accordance with guidelines reported by Soliman et al14 would also influence LMD rates. In this study, we report the risk and predictors of developing LMD in a consecutive case series of patients treated with HSRT for either surgical cavities or intact metastases. The objectives were to (i) identify clinical and dosimetric predictors of LMD, (ii) characterize the radiographic patterns of LMD, and (iii) determine if these patterns influence survival.
Methods
Patient and Treatment Data
This report is based on a retrospective review of a prospective database of patients treated with HSRT for intact or resected brain metastases between May 2009 and December 2014. The study was approved by the local institutional research board. Patient and treatment details reviewed included age, Karnofsky performance score (KPS), control of extracranial disease status, tumor histology, maximum tumor diameter, tumor location (supratentorial vs infratentorial), intact versus resected, total number of brain metastases at the time of HSRT, presence of dural involvement, and histologic sensitivity to radiotherapy. Histologies sensitive to radiotherapy included breast cancer, non–small cell lung cancer and small cell lung cancer. Radioresistant tumor histologies included melanoma, renal carcinoma, colorectal carcinoma, and sarcoma. Treatment-related variables included extent of resection, use of a targeted agent or immunotherapy (TA/I) at any point, date of LMD diagnosis, prior whole brain radiotherapy (WBRT), WBRT after LMD diagnosis, time from LMD diagnosis to death, and LC of the HSRT target. Targeted agents included tyrosine kinase inhibitors for patients with epidermal growth factor receptor driver mutations and breast cancer therapies such as trastuzumab, pertuzumab, lapatinib, and trastuzumab emtansine. Immunotherapy considered were immune checkpoint blockade agents, including pembrolizumab and nivolumab. Dosimetric factors included total dose, planning target volume (PTV), clinical target volume (CTV), and the brain minus cavity or gross tumor volume (GTV) for the 5 Gy (BMC-5Gy), 10 Gy (BMC-10Gy), and 15 Gy (BMC-15Gy) isodose volumes. To mitigate the potential confounding impact of tumor size, we created normalized volumes of interest for the isodose volumes by dividing by the PTV (eg, “Brain minus cavity-GTV / PTV”).
Hypofractionated Stereotactic Radiotherapy Technique
Our HSRT technique has previously been reported.15 In brief, patients were immobilized in a supine position with a 5-point thermoplastic mask. A simulation CT of the brain was obtained with a 1 mm slice thickness, and this was co-registered to a standardized T1-weighted contrast-enhanced volumetric MRI with a 1.5 mm slice thickness.
Patients were treated using a linear accelerator equipped with a 4–5 mm multileaf collimator, on board cone-beam CT image-guidance system, and a robotic couch (Elekta Axesse). For patients with resected brain metastases, we aimed to initiate radiotherapy 2 to 4 weeks after surgery to allow adequate time for surgical healing and postoperative cavity changes to stabilize.16
For intact brain metastases, the GTV was defined as enhancing tumor on contrast-enhanced T1-weighted MRI sequences. In the postoperative setting, the GTV encompassed the residual disease in the context of a subtotal resection (STR) and was not applicable in cases of a gross total resection (GTR). The CTV was delineated in adherence to international consensus contouring guidelines.14 For intact metastases, no CTV margin was applied. A PTV margin was then generated as a 2 mm isotropic expansion on the CTV. The standard at our institution is to deliver HSRT in 5 daily fractions, with 30 Gy being the most common total dose.
Patterns of LMD
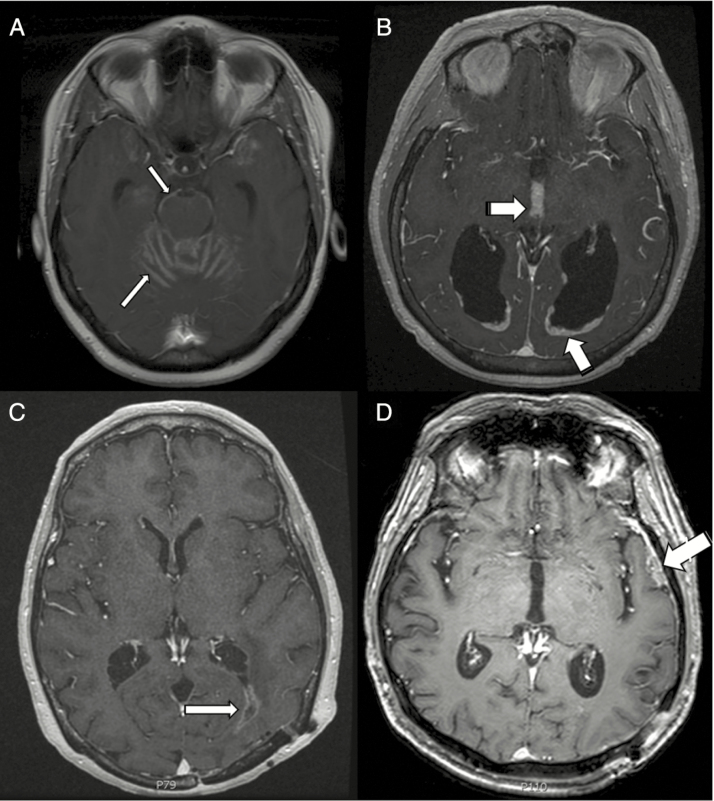
Patterns of LMD were determined radiographically based on the first MRI at which LMD was diagnosed. Patterns were assigned based on independent imaging review by 2 radiation oncologists and a neuroradiologist. Our review of the literature identified classical LMD (cLMD) as “enhancement of the cranial nerves, cisterns, cerebellar folia, and sulci or diffuse sugar-coating enhancement across the surface of the brain,” whereas nodular LMD (nLMD) was considered a “morphologically distinct pattern differentiated from distant brain parenchymal recurrence.” 7 In this report we further classified LMD into 4 patterns: focal classical, diffuse classical, focal nodular, and diffuse nodular6,7,17 (Fig. 1).
Fig. 1.
(A) An example of diffuse cLMD with enhancement of the cerebellar folia and brainstem (thin arrows). (B) An example of diffuse nLMD with periependymal and interpeduncular nodular enhancement (thick arrows). (C) Focal cLMD (thin arrow). (D) Focal nLMD (thick arrow).
Statistical Analysis
Descriptive statistics were generated for all abstracted data. Comparison of baseline demographic, tumor, and treatment characteristics between intact and cavity lesions was assessed using chi-square/Fisher’s exact test (as appropriate) for categorical and Student’s t-test for continuous covariates of interest. Potential predictors for the development of LMD were assessed by univariate and multivariable analyses using logistic regression. The outcome variables of interest included development of LMD and overall survival (OS). OS rates were calculated using the Kaplan–Meier product-limit method. The log-rank test was used in univariate analysis to assess potential prognostic factors for OS. The cumulative incidences of LMD rates were obtained with death as a competing risk. All P-values were 2-sided and for the statistical analyses, P < 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS v9.4 for Windows.
Results
Patient, Tumor, and Treatment Characteristics
We analyzed 235 patients with 320 intracranial metastases treated with HSRT, and the median follow-up was 13.4 months (range, 0.8–60 mo). The demographic, tumor, and treatment characteristics are summarized in Table 1. Nearly all patients (97.9%) had a KPS of ≥70, and 90.2% had between 1 and 4 brain metastases (range, 1–12). The distribution of intact metastases and surgical cavities were 183 (57.2%) and 137 (42.8%), respectively. For both intact metastases and cavities, the most common tumor histologies were lung (46.6%), breast (23.1%), and melanoma (8.4%). Compared with intact tumors, patients in the surgical cavity group were more likely to have single brain metastases, dural involvement, a larger maximum lesion diameter, and a larger PTV.
Table 1.
Summary of baseline demographic, tumor, and treatment characteristics
| Characteristic, n (%) | Entire Cohort | Intact Metastases | Cavities | P-value (Intact vs Cavities) |
|---|---|---|---|---|
| Target Lesions (n = 320) | Target Lesions (n = 183) | Target Lesions (n = 137) | ||
| Age, y (n = 235 patients) | 0.1861 | |||
| Median (range) | 63 (21 to 92) | 66 (21 to 92) | 62 (24 to 92) | |
| KPS (n = 235 patients) | 0.0341 | |||
| 100 | 25 (10.6) | 14 (12.50) | 11 (8.94) | |
| 90 | 105 (44.7) | 45 (40.18) | 60 (48.78) | |
| 80 | 71 (30.2) | 30 (26.79) | 41 (33.33) | |
| 70 | 29 (12.3) | 18 (16.07) | 11 (8.94) | |
| ≤60 | 5 (2.1) | 5 (4.46) | 0 (0) | |
| Control of extracranial disease (n = 235 patients) | 0.0894 | |||
| No | 113 (61.4) | 67 (67) | 46 (54.76) | |
| Yes | 71 (38.6) | 33 (33) | 38 (45.24) | |
| Not available | 51 | 12 | 39 | |
| Number of brain metastases (n = 235 patients) | <0.0001 | |||
| 1 | 134 (57.0) | 44 (39.29) | 90 (73.17) | |
| 2–4 | 78 (33.2) | 48 (42.86) | 30 (24.39) | |
| ≥5 | 23 (9.8) | 20 (17.86) | 3 (2.44) | |
| Histology | 0.3748 | |||
| Lung | 149 (46.6) | 89 (48.63) | 60 (43.80) | |
| Breast | 74 (23.1) | 44 (24.04) | 30 (21.90) | |
| Melanoma | 27 (8.4) | 18 (9.84) | 9 (6.57) | |
| Gastrointestinal | 21 (6.6) | 10 (5.46) | 11 (8.03) | |
| Renal | 20 (6.2) | 9 (4.92) | 11 (8.03) | |
| Other | 29 (9.1) | 13 (7.10) | 16 (11.68) | |
| Sensitivity to radiotherapy | 0.3131 | |||
| Sensitive | 247 (77.2) | 145 (79.23) | 102 (74.45) | |
| Resistant | 73 (22.8) | 38 (20.77) | 35 (25.55) | |
| Location | 0.7138 | |||
| Supratentorial | 242 (77.2) | 137 (74.86) | 105 (76.64) | |
| Infratentorial | 78 (24.8) | 46 (25.14) | 32 (23.36) | |
| Dural involvement | 0.0038 | |||
| No | 247 (77.19) | 152 (83.06) | 95 (69.34) | |
| Yes | 73 (22.81) | 31 (16.94) | 42 (30.66) | |
| Extent of resection (n = 136) | N/A | |||
| GTR | 123 (90.4) | N/Ab | 123 (90.44) | |
| STR | 13 (9.6) | 13 (9.56) | ||
| Max diameter, cm | <0.0001 | |||
| Median (range) | 2.3 (0.4 to 7.0) | 1.8 (0.4 to 7) | 3.3 (0.8 to 7) | |
| Mean | 2.6 | 2.0 | 3.28 | |
| PTV volume, cc | <0.0001 | |||
| Median (range) | 12.5 (0.4 to 175.2) | 7.0 (0.4 to 105) | 24.4 (5.0 to 175.2) | |
| Radiotherapy total dose, cGy (in 5 fractions) | 0.0731 | |||
| 2250–2750 | 64 (20.0) | 43 (23.50) | 21 (15.33) | |
| 3000 | 209 (65.3) | 110 (60.11) | 99 (72.26) | |
| 3250–3750 | 47 (14.7) | 30 (16.39) | 17 (12.41) | |
| Received targeted agent (n = 235) | 0.7250 | |||
| Yes | 75 (31.9) | 37 (33.04) | 38 (30.89) | |
| No | 160 (68.1) | 75 (66.96) | 85 (69.11) | |
| Time from surgical resection to radiotherapy, days (n = 137) | ||||
| Adjuvant HSRT, median (range) | 36 (17 to 175) | N/A | 36 (17 to 175) | N/A |
| Salvage HSRT, median (range) | 41.5 (22 to 657) | N/A | 41.5 (22 to 657) | N/A |
| Previous WBRT before developing LMD (n = 45) | 0.6598a | |||
| Yes | 7 (15.6) | 3 (21.43) | 4 (12.90) | |
| No | 38 (84.4) | 11 (78.57) | 27 (87.10) |
aFisher’s exact test was used. bN/A = not applicable.
Of the resected metastases, 123 (90%) had a GTR and 113 (82.5%) received adjuvant cavity radiotherapy with a median time from completion of surgery of 36 days (range, 17–71 days, with one patient receiving radiotherapy at 175 days as treatment was delayed until after chemotherapy). In the remaining 24 cases (17.5%), patients declined or were unable to receive adjuvant HSRT; however, subsequent salvage HSRT was delivered at the time of local progression. The median time from surgery to salvage HSRT in that subset was 42 days (range, 22–657 days).
Local control rates for the intact metastases cohort was 92%, 78%, and 65% at 6 months, 12 months, and 24 months, respectively. For the cavity cohort, LC rates at the same time points were 91%, 84%, and 74%. Rates of symptomatic radiation necrosis were 9% for intact metastases and 6% for the cavity cohort.18,19
Overall Population
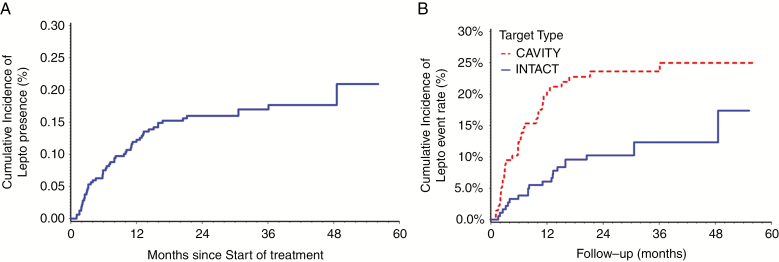
The overall population was 235 patients with 320 intracranial metastases. Overall, the cumulative incidence of LMD after HSRT was 19.2% (n = 45; Fig. 2). The median time to LMD was 7.7 months from the date HSRT was completed. Of these patients, the most common pattern of LMD observed was diffuse nodular (44.4%) followed by diffuse classic (26.7%), focal nodular (15.6%), then focal classic (13.3%). Nodular LMD was more common than cLMD (60% versus 40%), and diffuse presentations were more common than focal (71.1% vs 28.9%). The overall rates of developing LMD at 1 year and 2 years after HSRT were 12% and 16%, respectively (Table 2). The majority of patients (84.4%) who developed LMD did not have prior WBRT.
Fig. 2.
(A) The overall cumulative incidence of LMD over time following HSRT. (B) The cumulative incidence of LMD over time following HSRT for intact vs cavity metastases.
Table 2.
Summary of HSRT outcomes
| Characteristic | Patients, N (%) |
|---|---|
| Overall incidence of LMD (n = 235) | |
| Yes | 45 (19.2) |
| No | 190 (80.8) |
| Incidence of LMD for patients with only intact metastases (n = 183) | |
| Yes | 22 (12.0) |
| No | 161 (88.0) |
| Incidence of LMD for patients with postoperative cavities (n = 137) | |
| Yes | 33 (24.1) |
| No | 104 (75.9) |
| Pattern of LMD (n = 45 targets) | |
| Diffuse Classic | 12 (26.7) |
| Diffuse Nodular | 20 (44.4) |
| Focal Classic | 6 (13.3) |
| Focal Nodular | 7 (15.6) |
| Nodular | 27 (60.0) |
| Classic | 18 (40.0) |
| Overall rate of developing LMD, event rate (95% CI) | |
| 1 year after HSRT | 12% (6% to 18%) |
| 2 years after HSRT | 16% (7% to 25%) |
| Rate of LMD for patients with only intact metastases | |
| 1 year after HSRT | 6% (0% to 13%) |
| 2 years after HSRT | 10% (0% to 24%) |
| Rate of LMD for patients with postoperative cavities | |
| 1 year after HSRT | 20% (11% to 29%) |
| 2 years after HSRT | 24% (12% to 35%) |
| Death without developing LMD | 143 (60.9) |
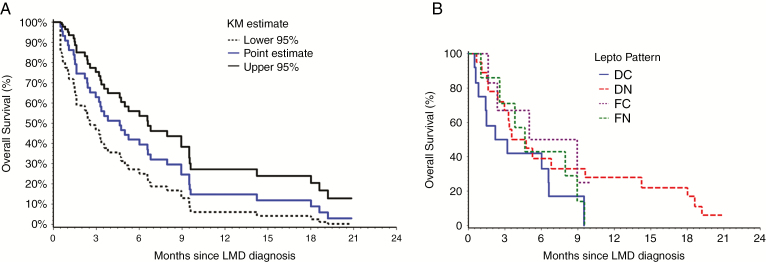
From the date of LMD diagnosis, the median follow-up was 3.8 months (range, 0.2–20.8 mo) and the 6-month and 12-month OS rates for the entire cohort (n = 45) were 42% and 15%, respectively (Fig. 3). There were no significant differences in OS rates between the 4 patterns of LMD (P = 0.203; Fig. 4), or between nLMD versus cLMD (P = 0.267). For patients with nLMD, the median follow-up was 3.8 months (range, 0.2–20.8 mo) and 6-month and 12-month OS rates were 40% and 20%, respectively. For cLMD, the median follow-up was 4.1 months (range, 0.5–10 mo) and the 6-month and 12-month OS rates were 44% and 0%, respectively. Patients who received WBRT upon diagnosis of LMD (n = 34) had a median survival of 5.3 months (95% CI: 3.2, 8 mo), compared with 1.6 months (95% CI: 1.1, 8.9 mo) for patients who did not receive WBRT (n = 8); however, this difference did not reach statistical significance (P = 0.111).
Fig. 3.
(A) The overall survival from time of leptomeningeal diagnosis for the whole cohort (45 patients who developed LMD). (B) OS across the 4 patterns of LMD.
Fig. 4.
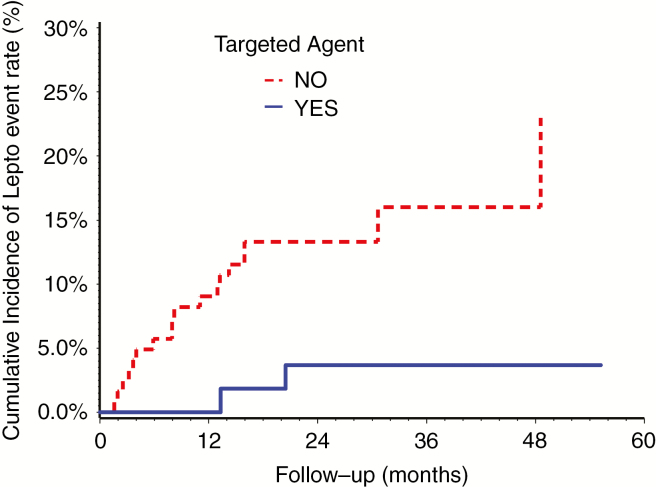
Cumulative incidence of LMD rate for patients with or without TA/I.
On univariate analysis, statistically significant predictors for LMD included controlled extracranial disease (odds ratio [OR] = 2.245, P = 0.024), surgical resection (OR = 2.32;, P = 0.005), increasing KPS score (OR = 1.040, P = 0.022), increasing maximum tumor diameter (OR = 1.332, P = 0.011), and increasing PTV (OR = 1.013, P = 0.036). On multivariable analysis, surgical resection was the only statistically significant predictor for LMD (OR = 2.30, 95% CI: 1.24, 4.29, P = 0.008). Of note, PTV size and volume of normal brain (minus CTV) receiving 5 Gy, 10 Gy, or 15 Gy did not impact LMD rates on multivariable analysis.
Intact Metastases
There were 112 patients with 183 intracranial metastases. For patients with only intact metastases, there were 22 cases (12%) of LMD (Fig. 2), and the rates of developing LMD metastases at 1 year and 2 years were 6% and 10%, respectively. Thirteen of these cases were cLMD and 9 were nLMD. On univariate analysis, only the use of a TA/I was associated with a reduced risk of LMD (OR = 0.178, 95% CI: 0.04, 0.79; P = 0.023), and this remained significant on multivariable analysis.
Cavities
There were 123 patients with 137 resected metastases. For patients with a resected brain metastasis, there were 33 cases (24.1%) of LMD (Fig. 1), and the rates of LMD at 1 year and 2 years were 20% and 24%, respectively. Nodular LMD was more common than cLMD, which was observed in 24 cases (72.7%). A diffuse pattern was more common than focal, which was observed in 25 cases (75.8%). On univariate analysis, statistically significant predictors for LMD included sensitivity to radiotherapy (OR = 2.36, P = 0.038), control of extracranial disease (OR = 2.88, P = 0.047), and BMC-15 Gy (OR = 1.01, P = 0.042). On multivariable analysis, the only significant predictor for LMD was a histology sensitive to radiotherapy (OR = 2.35, 95% CI: 1.04, 5.35; P = 0.041). There was a trend for increasing maximum preoperative tumor diameter as a predictor for LMD, but this was not statistically significant (OR = 1.381, P = 0.076).
Discussion
Our crude rate of LMD was 24.1% for cavities and 12.0% for intact metastases. This is consistent with previously reported LMD rates after cavity SF-SRS that range from 6.3% to 24.0%.20–23 On multivariable analysis, we observed that resected brain metastases treated with postoperative HSRT were at a greater risk of developing LMD compared with intact metastases treated with HSRT (hazard ratio = 2.12, P = 0.01). The underlying pathophysiological mechanism may be associated with intraoperative tumor dissemination from the anatomic disruption of the meninges.24 When classifying the pattern of LMD according to focal nodular versus diffuse nodular versus focal classic versus diffuse classic, or according to simply nodular versus classic, we observed no impact on OS rates, which were uniformly poor.
Compared with the intact metastases group, patients with surgical cavities were more likely to have single brain metastases (vs multiple), dural involvement, and a larger maximum lesion diameter and PTV. While KPS, increasing maximum tumor diameter, and increasing PTV were significant predictors of LMD on univariate analysis, only surgical cavity was statistically significant on multivariable analysis. Therefore, the difference in the rate of LMD observed between the surgical cavity and intact cohorts is not significantly impacted by these factors.
With respect to the literature, there is consistency with other studies evaluating predictors of LMD after either SF-SRS and/or HSRT. We summarize the 4 studies identified in the literature that report predictors for LMD on multivariable analysis24–27 in the Supplementary Material. Consistent with our findings, 2 studies reported surgical resection as a risk factor for LMD, and 3 observed breast cancer histology as predictive. Of note, Johnson et al also reported on a mixed cohort of patients with 112 surgical cavities and 218 intact metastases treated with SF-SRS. Although the study did not differentiate between cLMD and nLMD, similar to our findings they observed a greater rate of LMD for cavities compared with intact metastases. Considered alongside our findings, treatment with HSRT does not appear to influence the risk of LMD despite the higher biologically effective dose, addition of a PTV margin, and a more generous meningeal margin for surgical cavity targets.
We observed that nLMD was more frequent after surgery, compared with cLMD, and this is in agreement with 2 notable recent investigations.23,27 Prabhu et al conducted a multi-institutional retrospective study of 147 patients who developed LMD following SRS/HSRT to surgical cavities in patients with at least one resected brain metastasis and no prior WBRT. They reported that nLMD (57%) was more common than cLMD (43%) and that survival was prolonged in LMD patients who received salvage radiotherapy (WBRT or focal radiotherapy) versus no further treatment (median 6.4 mo vs 0.9 mo, respectively; P < 0.001). Our results showed a trend toward improved survival following salvage WBRT in LMD patients, but this was not statistically significant (5.2 mo vs 1.6 mo; P = 0.098). Additionally, Prabhu et al reported patients with nLMD survived a median 8.2 months versus 3.3 months for those with cLMD following salvage treatment (P < 0.001). However, unlike Prabhu et al, we did not observe any survival difference based on subtype of LMD (Fig. 3B). The smaller sample size in our study and the different patient populations, notably the inclusion of only postoperative LMD patients in the study by Prabhu et al, may explain this discrepancy. We submit that the prognostic implication of LMD subtype warrants further rigorous analysis with consistent imaging follow-up before firm conclusions can be drawn.
In the retrospective single-institution analysis by Cagney et al the risk of LMD and pachymeningeal disease (PD) was evaluated in 1188 brain metastases patients. Of these patients, 318 were treated with surgery followed by postoperative radiotherapy and 870 were treated with radiotherapy alone. It is important to note that the focus of their investigation was the 318 postoperative patients and that they defined PD as “nodular enhancing tumors stemming from the pachymeninges, with no involvement of the overlying calvarium to suggest bony involvement with secondary extension into the pachymeninges.” By definition, PD and nLMD are distinct in their involvement of the pachymeninges and leptomeninges, respectively. However, these nuances are generally indistinguishable on imaging alone, and therefore in this study we considered these to represent the same radiographic entity as nLMD. Cagney et al observed that 36 of the 318 (11.3%) surgical patients developed nLMD, while no patients in the radiation alone cohort (N = 870) developed nLMD. The rate of cLMD was similar between the surgical (35 of 318) and the radiation alone patients (68 of 870) (data obtained from communication with study author). Similar to Cagney et al, we observed a higher rate of nLMD in surgical compared with intact metastases patients (19.5% versus 8.0%, respectively; P = 0.02). In contrast, Cagney et al observed no cases of nLMD in their intact metastases group, whereas nLMD occurred in both of our cohorts. This may reflect inherent selection bias given that these are distinct non-randomized study populations and/or the ongoing challenge in radiographically classifying LMD. Ultimately we need further data to validate these patterns of failure, and there needs to be a consensus guideline specific to the classification of LMD.
We report favorable LC rates with HSRT18 compared with historic outcomes for SF-SRS, yet LMD rates remain high particularly for postoperative patients, and strategies to reduce LMD risk are in need. Preoperative SRS appears to be a promising strategy, and uncontrolled comparative series suggest lower LMD rates than postoperative radiotherapy.28–30 Currently, at least 2 single-arm phase II trials are evaluating preoperative SRS (ClinicalTrials.gov identifiers: NCT03398694 and NCT02514915), and 1 phase III trial (ClinicalTrials.gov identifiers: NCT03741673) of preoperative versus postoperative SRS is actively accruing.
Our finding that patients in the intact metastases cohort receiving TA/I had a lower rate of LMD (Fig. 4) suggests a potential protective effect with these agents and an alternative approach to this problem. While the evidence supporting TA/I as a treatment for patients with established intracranial LMD continues to emerge,31 there is little known regarding the efficacy of TA/I in preventing or extending the time to the development of LMD. Across tumor sites, several clinical trials are actively recruiting patients to examine the role of TA/I in LMD, including a phase II trial evaluating the use of nivolumab and ipilimumab in patients with LMD from various primary histologies (ClinicalTrials.gov Identifier: NCT02939300). Recently, a pre-planned secondary analysis of the phase III AURA3 randomized trial reported on the efficacy of osimertinib versus platinum-pemetrexed chemotherapy with respect to those patients with CNS metastases.32 Patients in the osimertinib arm experienced a significant reduction in the rates of CNS progression. Furthermore, in the 7 patients in the osimertinib arm with upfront LMD at the time of treatment, there were no cases of CNS progression. Therefore, these agents may be a means of improving intracranial control in eligible patients and create an opportunity for a combined multimodality approach, whereby radiographically detectable sites of LMD are treated with focal SF-SRS or HSRT in those scheduled to receive TA/I. Prabhu et al also reported on the delivery of focal salvage radiotherapy to nLMD and found that patients treated in this manner had significantly longer OS compared with those treated with WBRT (median OS of 12.5 mo vs 4.4 mo, respectively; P < 0.001).23 As we further our understanding of the role of TA/I in the treatment of LMD, it is also critical to determine the ideal sequencing of these drugs with radiotherapy to optimize response rates while minimizing toxicity. There is an urgent need to confirm these promising strategies with clinical trials given the limited efficacy of current therapies for LMD, including WBRT.
Upfront WBRT may be a strategy to reduce rates of LMD in higher-risk postoperative patients; however, in the randomized controlled trials evaluating postoperative SRS compared with WBRT, the outcomes do not suggest that WBRT influenced the rate of LMD.12,33 This is reinforced by a recently presented abstract of a phase III trial of WBRT versus observation in patients who have received local treatment for 1–4 melanoma brain metastases. Fogarty et al reported no statistically significant difference in distant brain failure with the addition of adjuvant WBRT.34 In our multivariate analysis, low dose exposure and PTV volume did not influence the risk of LMD. Therefore, we would contend that upfront postoperative WBRT is unlikely to be a preferred alternative to cavity-directed radiotherapy, especially given the adverse neurocognitive profile.35,36
The present report has several notable strengths. First, this is one of the largest series of patients consistently receiving 5 fraction HSRT for indications that are reflective of clinical practice (eg, postoperative cavities, large intact metastases). Second, our cohort includes all consecutive patients treated with HSRT during the study period, rather than only examining patients who have developed LMD as other studies have done. This allowed us to determine the event rate and identify predictors of LMD. Third, our institution employs regular (every 2–3 mo) 3D volumetric MRI follow-up, which enhances the reliability of diagnosing LMD. Finally, we had neuroradiology review of all scans to ensure accuracy with respect to diagnosis and classification. The limitations of this study include the retrospective nature of the study, limited sample size and event rate, and that we did not differentiate between symptomatic LMD and asymptomatic LMD.
Conclusion
The risk of LMD is significantly greater in patients who have undergone surgical resection for brain metastases despite postoperative cavity HSRT. In patients treated with HSRT for intact metastases, the use of TA/I reduced the risk of LMD. Survival remains poor for patients with LMD, and no differences were observed between the different patterns of LMD. Novel, multimodality strategies are required to mitigate the risk of LMD, and clinical trials evaluating these approaches are in need.
Supplementary Material
Funding
None.
Conflict of interest statement.
Dr Sahgal’s disclosures: Advisor/consultant with Abbvie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board). Ex officio Board Member: International Stereotactic Radiosurgery Society (ISRS). Past educational seminars with Elekta AB, Accuray Inc, Varian (CNS Teaching Faculty), BrainLAB, Medtronic Kyphon. Research grant with Elekta AB. Travel accommodations/expenses by Elekta, Varian, BrainLAB. Dr Sahgal also belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia. Dr Ruschin is the co-inventor of and owns associated intellectual property specific to the image-guidance system on the Gamma Knife Icon.
Authorship statement.
Design and conception: AS, HS, TN. Data analysis and interpretation: TN, HS, EA, AS, PM, CH. Manuscript writing: TN, AS, HS, EA, MR, ZH, SM. Revisions: TN, AS, HS, EA, JD, SM, CT, ZH, CH, PM, MR, JP.
References
- 1. Chamberlain M, Soffietti R, Raizer J, et al. . Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16(9):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Debnam JM, Mayer RR, Chi TL, et al. . Most common sites on MRI of intracranial neoplastic leptomeningeal disease. J Clin Neurosci. 2017;45:252–256. [DOI] [PubMed] [Google Scholar]
- 3. Mack F, Baumert BG, Schäfer N, et al. . Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev. 2016;43:83–91. [DOI] [PubMed] [Google Scholar]
- 4. Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38(1):51–57. [DOI] [PubMed] [Google Scholar]
- 5. Lee YY, Tien RD, Bruner JM, De Pena CA, Van Tassel P. Loculated intracranial leptomeningeal metastases: CT and MR characteristics. AJNR Am J Neuroradiol. 1989;10(6):1171–1179. [PMC free article] [PubMed] [Google Scholar]
- 6. Chamberlain M, Junck L, Brandsma D, et al. . Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner BE, Prabhu RS, Burri SH, et al. . Nodular leptomeningeal disease—a distinct pattern of recurrence after post-resection stereotactic radiosurgery for brain metastases: a multi-institutional study of inter-observer reliability. Int J Radiat Oncol Biol Phys. 2018;102(3):e363–e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minniti G, Scaringi C, Paolini S, et al. . Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142–1148. [DOI] [PubMed] [Google Scholar]
- 9. Minniti G, Esposito V, Clarke E, et al. . Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys. 2013;86(4):623–629. [DOI] [PubMed] [Google Scholar]
- 10. Lockney NA, Wang DG, Gutin PH, et al. . Clinical outcomes of patients with limited brain metastases treated with hypofractionated (5×6Gy) conformal radiotherapy. Radiother Oncol. 2017;123(2):203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shaw E, Scott C, Souhami L, et al. . Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. 1996;34(3):647–654. [DOI] [PubMed] [Google Scholar]
- 12. Brown PD, Ballman KV, Cerhan JH, et al. . Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahajan A, Ahmed S, McAleer MF, et al. . Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soliman H, Ruschin M, Angelov L, et al. . Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2018;100(2):436–442. [DOI] [PubMed] [Google Scholar]
- 15. Al-Omair A, Soliman H, Xu W, et al. . Hypofractionated stereotactic radiotherapy in five daily fractions for post-operative surgical cavities in brain metastases patients with and without prior whole brain radiation. Technol Cancer Res Treat. 2013;12(6):493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alghamdi M, Hasan Y, Ruschin M, et al. . Stereotactic radiosurgery for resected brain metastasis: cavity dynamics and factors affecting its evolution. J Radiosurg SBRT. 2018;5(3):191–200. [PMC free article] [PubMed] [Google Scholar]
- 17. Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5(5):443–452. [DOI] [PubMed] [Google Scholar]
- 18. Myrehaug S, Soliman H, Ruschin ME, Tseng CL, Tsao M, Sahgal A. Clinical outcomes for frameless image-guided stereotactic radiation therapy to intact brain metastases in five daily hypofractionated treatments. Int J Radiat Oncol Biol Phys. 2017;99:E96–E97. [Google Scholar]
- 19. Soliman H, Myrehaug S, Tsao M, et al. . Outcomes for image-guided frameless linear accelerator-based hypofractionated stereotactic radiotherapy in 5 daily fractions to the surgical cavity after resection of brain metastases. Int J Radiat Oncol Biol Phys. 2017;99(2):E110. [Google Scholar]
- 20. Steinmann D, Maertens B, Janssen S, et al. . Hypofractionated stereotactic radiotherapy (hfSRT) after tumour resection of a single brain metastasis: report of a single-centre individualized treatment approach. J Cancer Res Clin Oncol. 2012;138(9):1523–1529. [DOI] [PubMed] [Google Scholar]
- 21. Iwai Y, Yamanaka K, Yasui T. Boost radiosurgery for treatment of brain metastases after surgical resections. Surg Neurol. 2008;69(2):181–186; discussion 186. [DOI] [PubMed] [Google Scholar]
- 22. Robbins JR, Ryu S, Kalkanis S, et al. . Radiosurgery to the surgical cavity as adjuvant therapy for resected brain metastasis. Neurosurgery. 2012;71(5):937–943. [DOI] [PubMed] [Google Scholar]
- 23. Prabhu RS, Turner BE, Asher AL, et al. . A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro Oncol. 2019;21(8):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson MD, Avkshtol V, Baschnagel AM, et al. . Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94(3):537–543. [DOI] [PubMed] [Google Scholar]
- 25. Press RH, Zhang C, Chowdhary M, et al. . Hemorrhagic and cystic brain metastases are associated with an increased risk of leptomeningeal dissemination after surgical resection and adjuvant stereotactic radiosurgery. Neurosurgery. 2018;0(0):1–10. [DOI] [PubMed] [Google Scholar]
- 26. Huang AJ, Huang KE, Page BR, et al. . Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neurooncol. 2014;120(1):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cagney DN, Lamba N, Sinha S, et al. . Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol. 2019;5(5):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marchan EM, Peterson J, Sio TT, et al. . Postoperative cavity stereotactic radiosurgery for brain metastases. Front Oncol. 2018;8:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asher AL, Burri SH, Wiggins WF, et al. . A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88(4):899–906. [DOI] [PubMed] [Google Scholar]
- 30. Patel KR, Burri SH, Asher AL, et al. . Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery. 2016;79(2):279–285. [DOI] [PubMed] [Google Scholar]
- 31. Thomas KH, Ramirez RA. Leptomeningeal disease and the evolving role of molecular targeted therapy and immunotherapy. Ochsner J. 2017;17(4):362–378. [PMC free article] [PubMed] [Google Scholar]
- 32. Wu YL, Ahn MJ, Garassino MC, et al. . CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: data from a randomized Phase III trial (Aura3). J Clin Oncol. 2018;36:2702–2709. [DOI] [PubMed] [Google Scholar]
- 33. Kępka L, Tyc-Szczepaniak D, Bujko K, et al. . Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: results from a randomized trial. Radiother Oncol. 2016;121(2):217–224. [DOI] [PubMed] [Google Scholar]
- 34. Fogarty G, Dolven-Jacobsen K, Morton RL, et al. . Phase 3 international trial of adjuvant whole brain radiotherapy (WBRT) or observation (Obs) following local treatment of 1–3 melanoma brain metastases (MBMs). J Clin Oncol. 2019;37:15 supp. [Google Scholar]
- 35. Brown PD, Jaeckle K, Ballman KV, et al. . Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang EL, Wefel JS, Hess KR, et al. . Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 37. Keller A, Doré M, Cebula H, et al. . Hypofractionated stereotactic radiation therapy to the resection bed for intracranial metastases. Int J Radiat Oncol Biol Phys. 2017;99(5):1179–1189. [DOI] [PubMed] [Google Scholar]
- 38. Atalar B, Modlin LA, Choi CY, et al. . Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87(4):713–718. [DOI] [PubMed] [Google Scholar]
- 39. Strauss I, Corn BW, Krishna V, et al. . Patterns of failure after stereotactic radiosurgery of the resection cavity following surgical removal of brain metastases. World Neurosurg. 2015;84(6):1825–1831. [DOI] [PubMed] [Google Scholar]
- 40. Kaidar-Person O, Deal AM, Anders CK, et al. . The incidence and predictive factors for leptomeningeal spread after stereotactic radiation for breast cancer brain metastases. Breast J. 2018;24(3):424–425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.