Figure 1.
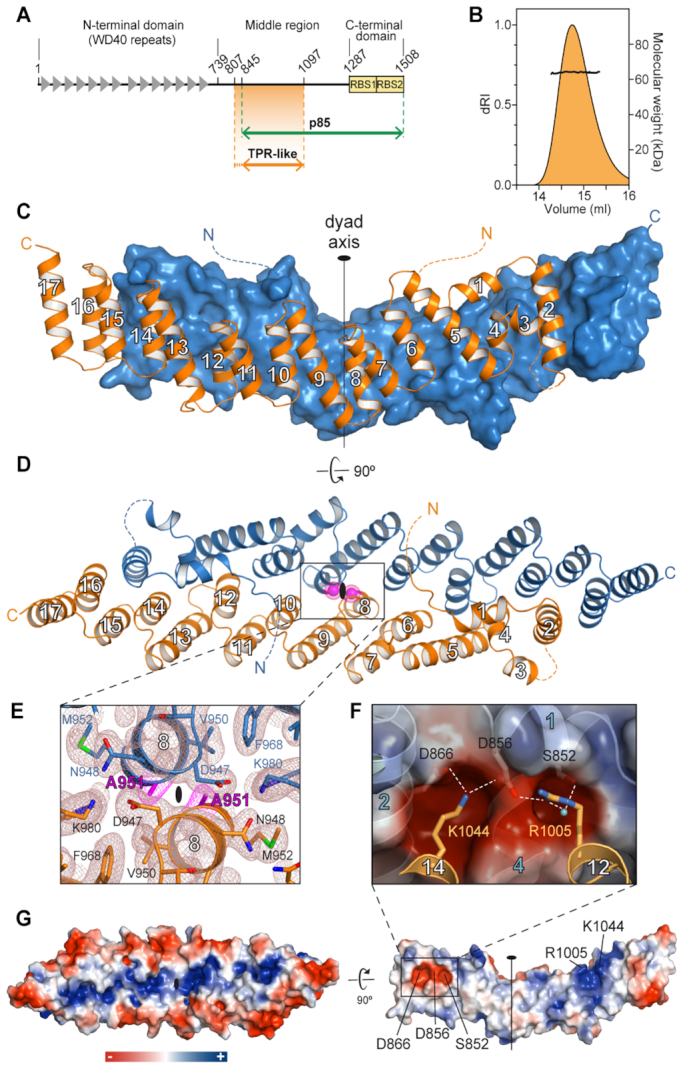
Crystal structure of Gemin5 TPR-like dimerization module. (A) Scheme of Gemin5 protein. WD40 repeats and RBS domains are indicated with gray triangles and yellow boxes, respectively. The p85 fragment and the TPR-like domain are indicated with green and orange arrows, respectively. (B) SEC-MALS analysis proves that G5-TPR is a dimer in solution, with a molecular weight of 64 kDa (±0.01%). (C) Structure of G5-TPR dimer with the subunit at front depicted in orange cartoon and the subunit at the back shown in blue surface representation. (D) Perpendicular view of (C) with both subunits represented in cartoon. The position of residues A951 in both subunits across the dyad axis are represented with magenta spheres. Dashed lines indicate regions not seen in the electron density maps. (E) Detail of the intersubunit interactions across the dyad axis and localization of residue A951. The 2Fobs− Fcalc electron density map at 1σ is shown in brown mesh. Residue A951 is highlighted in magenta. (F) Electrostatic interactions between residues D866 and D856 in one subunit, and R1005 and K1044 in the other subunit. A water molecule bridging the side chains of R1005 and S852 is shown as a cyan sphere. (G) Surface representation of the protein dimer (left) and of the dimerization interface of one of the subunits (right), colored according to the electrostatic potential. Blue and red contours begin at +2.5 and −2.5 kT, respectively.
