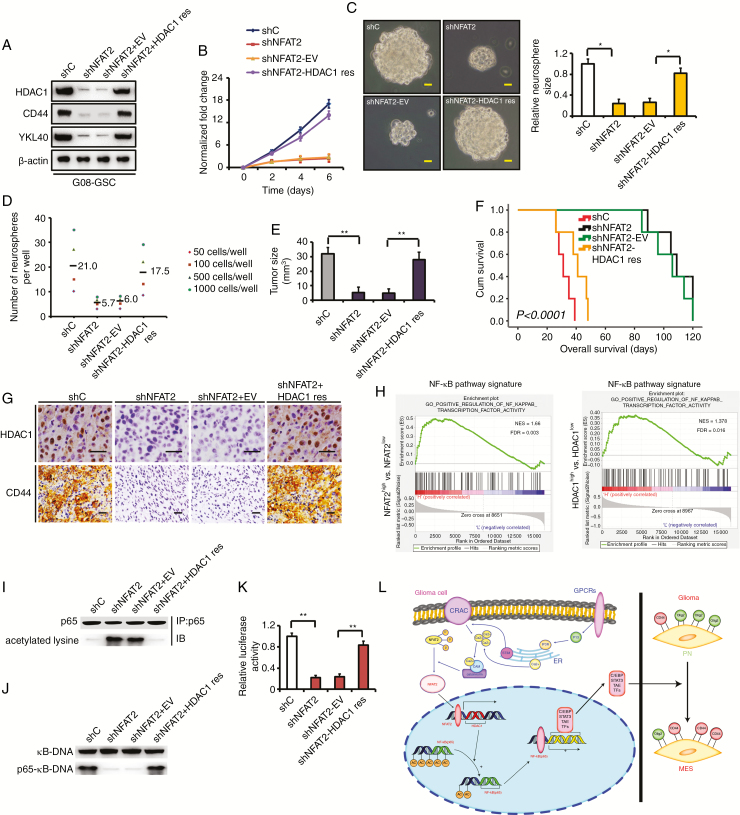
Fig. 6.
Rescue of HDAC1 in NFAT2-silenced GSCs partially restores clonogenicity in vitro and in vivo. (A) Rescuing HDAC1 in NFAT2-silenced G08 restores the expression of CD44 and YKL40 in vitro. (B) Rescue of HDAC1 partially restores proliferation compared with an empty vector control in NFAT2-silenced G08. (C) Reexpression of HDAC1 rescues neurosphere growth compared with an empty vector control in NFAT2-silenced G08. Scale bar = 25 μm. (D) Neurosphere formation after HDAC1 is rescued in NFAT2-silenced G08. Neurosphere formation capacity is significantly increased after HDAC1 rescue. (E) NFAT2 silencing in G08 inhibits the tumor growth in vivo. Rescuing HDAC1 in NFAT2-silenced G08 reverts tumor growth in vivo. (F) NFAT2 silencing in G08 prolongs the survival of tumor-bearing mice. Reexpression of HDAC1 in NFAT2-silenced G08 reverts the survival time. (G) Rescuing HDAC1 in NFAT2-silenced G08 reverts the expression of CD44 in vivo. Scale bar = 25 μm. (H) Gene set enrichment analysis plots of NF-κB pathway signatures in high NFAT2/HDAC1 expression versus low NFAT2/HDAC1 expression TCGA gliomas. Normalized enrichment score (NES) and false discovery rate (FDR) are shown in the plot. (I–K) HDAC1 reexpression in NFAT2-silences G08 inhibits hyperacetylation of p65 (I), restores the ability of p65 to bind κB-DNA (J), and recovers NF-κB–dependent transcriptional activity (K). (L) A working model of mesenchymal transition mediated by NFAT2/HDAC1/NF-κB pathway in gliomas. Results are presented as mean ± SD of triplicate samples from 3 independent experiments. *P < 0.05, **P < 0.01.