Figure 4.
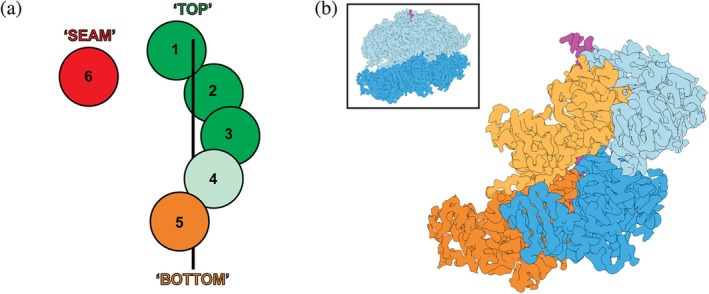
The nucleotide state of a AAA+ subunit depends on its position within the spiral staircase. (a) Cartoon representation of the spiral staircase arrangement with five substrate‐engaged and one dis‐engaged subunit, depicting their nucleotide states. ATP‐bound protomers are colored green, with a lighter green in position 4 indicating some accounts of ADP and potential hydrolysis in this subunit. The substrate‐disengaged “seam” subunit is colored red to represent an ADP‐bound or apo state, while the “Bottom” subunit is shown in orange to indicate a mostly ADP‐bound state, with some accounts of ATP in this position. (b) EM density for the “KC‐2” state of ClpB (EMDB:4625, complete hexamer shown in the inset with D1 domains in light blue and D2 domains in dark blue) reveals the slanted conformation observed in double ring AAA+ motors. The D1 and D2 domains are shown in light and dark blue for protomer 3, and in light and dark orange for protomer 4, highlighting how the D1 domain of a subunit overlaps with the D2 domain of the counterclockwise‐next neighbor
