Figure 6.
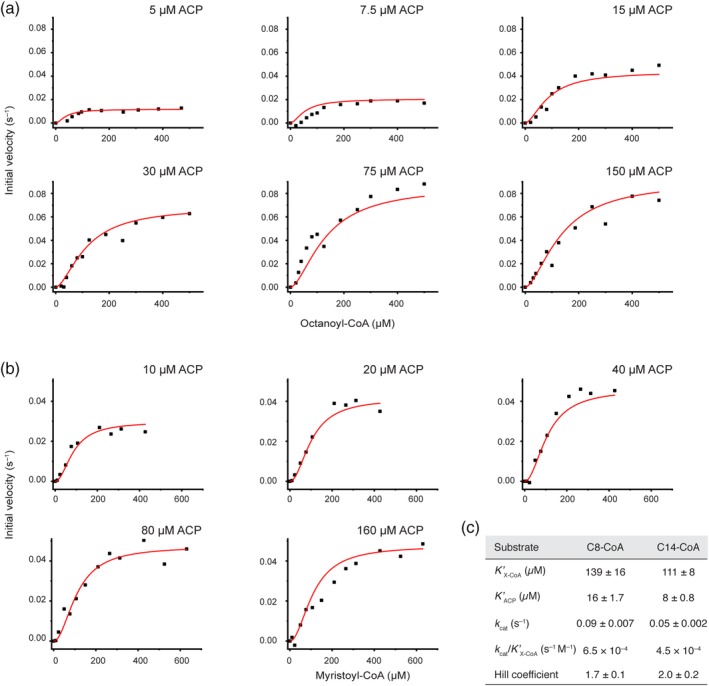
Comprehensive analysis of the KS‐mediated transfer of octanoyl and myristoyl moieties. (a) Initial velocities plotted against octanoyl‐CoA (C8‐CoA) concentrations at six fixed ACP concentrations. (b) Initial velocities plotted against myristoyl‐CoA (C14‐CoA) concentrations at five fixed ACP concentrations. All data series were fit globally with the Hill equation due to the sigmoidal shape. (c) Absolute kinetic parameter derived from the respective global fits for octanoyl‐CoA (C8‐CoA) and myristoyl‐CoA (C14‐CoA), respectively. No parameters constraints were imposed during curve fitting. The constant K′ of the Hill equation is related to the Michaelis constant K m, but also contains terms related to the effect of substrate occupancy at one site on the substrate affinity of the other site. ACP, acyl carrier protein; KS, ketosynthase
