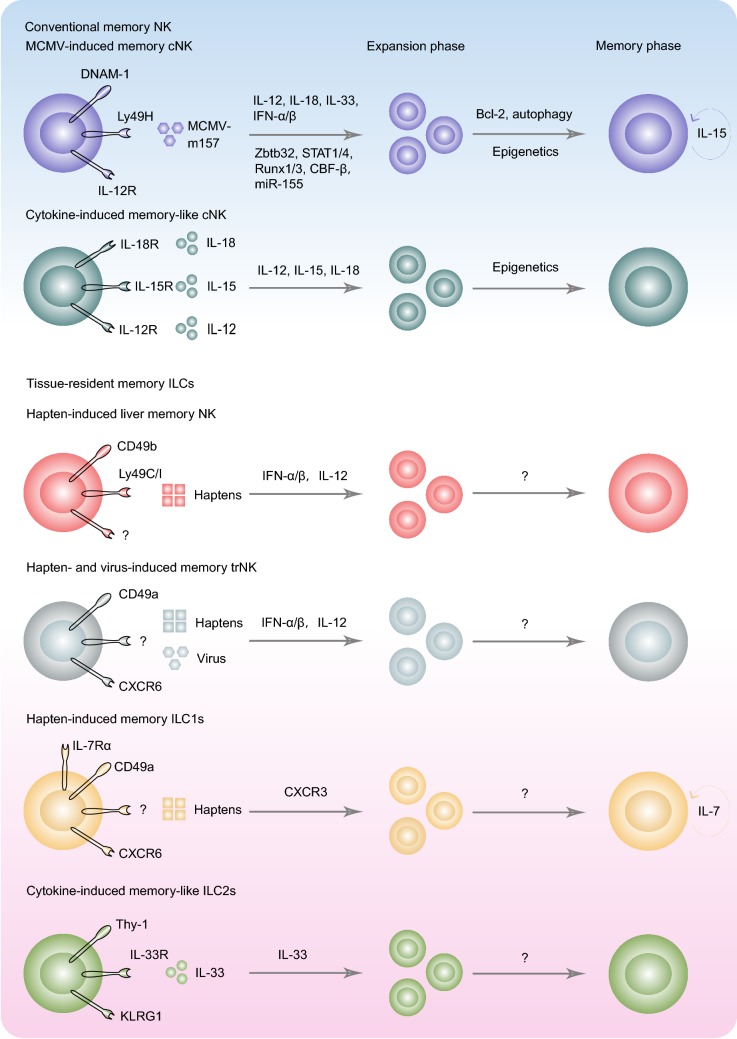
Figure 1.
Memory formation and long-term maintenance of tissue-resident memory ILCs and conventional memory NK cells. Memory ILCs comprise MCMV- or IL-12/15/18-induced circulating populations, and hapten-, virus-, or IL-33-induced tissue-resident subsets. Antigen-specific cNK cells respond to MCMV via three signals, including recognition receptor Ly49H, co-stimulatory molecule DNAM-1, and pro-inflammatory cytokines. Effector Ly49H+ NK cells go through the expansion phase under the control of transcription factors, such as Zbtb32, STAT1/4, Runx1/3, and CBF-β. A small fraction of effector Ly49H+ NK cells can survive against apoptosis to generate a memory pool. Memory Ly49H+ NK cells circulate in the blood and populate throughout the body. Cytokine-induced non-specific memory cNK cells also show similar migration patterns. Apart from these circulating memory NK cells, hapten-induced memory NK cells and ILC1s exhibit long-term residency in the liver. CD49b+ cNK cells may recognize haptens or hapten-peptide complexes dependently on the Ly49C/I receptors. Inflammatory cytokines IFN-α/β and IL-12 drive the memory formation of hapten-specific liver NK cells. Of note, key factors contributing to the retention of memory CD49b+ cNK cells have not been determined. CD49a+CXCR6+ NK cells can mediate recall responses to different viruses and haptens; however, the associated recognition receptors remain largely unknown. Both CD49b+Ly49C/I+ and CD49a+CXCR6+ NK cells prefer to reside in the liver after memory formation. Haptens also induce memory IL-7Rα+ ILC1 generation in draining LNs. The LN-derived memory ILC1s selectively maintain their longevity in the liver via CXCR6 and IL-7. In addition, IL-33-experienced lung ILC2s exhibit higher responsiveness to IL-33 and IL-25, confirming their memory-like features