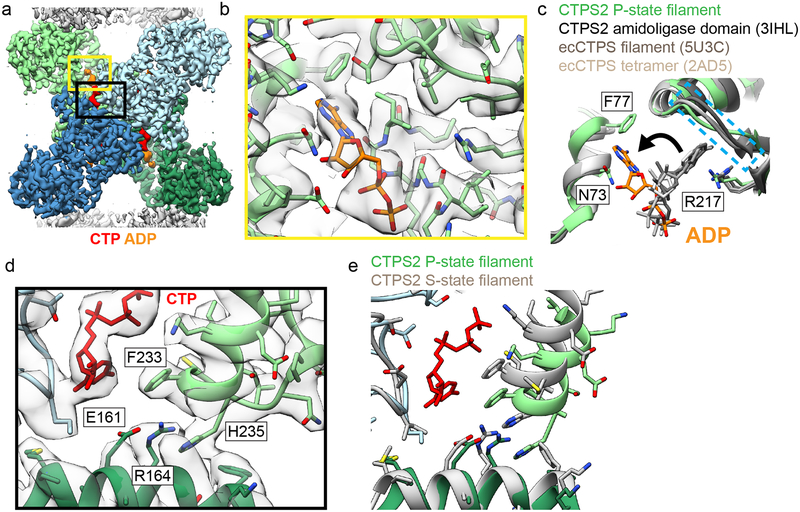
Fig. 2: Product binding and changes in the tetramer interface in CTPS2 structures.
(a) CTPS2 P-state tetramer, colored by monomer. (b) Zoomed-in view of the yellow box in (a), showing ADP (orange) bound in a novel conformation. Cryo-EM density is shown in transparent grey. (c) Comparison of ADP conformations in the CTPS2 P-state filament (color) with existing ADP-bound CTPS structures (grey). ADP in the P-state filament is packed between residues F77 and N73. In other CTPS structures, ADP is bound to a pocket formed by R217 and lid residues 244–250 (dashed blue box). (d) Zoomed-in view of the black box in (a), showing the CTP binding site. (e) Comparison of the tetramer interface around the CTP binding site in the P-state (blue and green) and S-state (grey) structures.