Figure 1. Transfer assays to study ligands required for cell-cell communication by EVs.
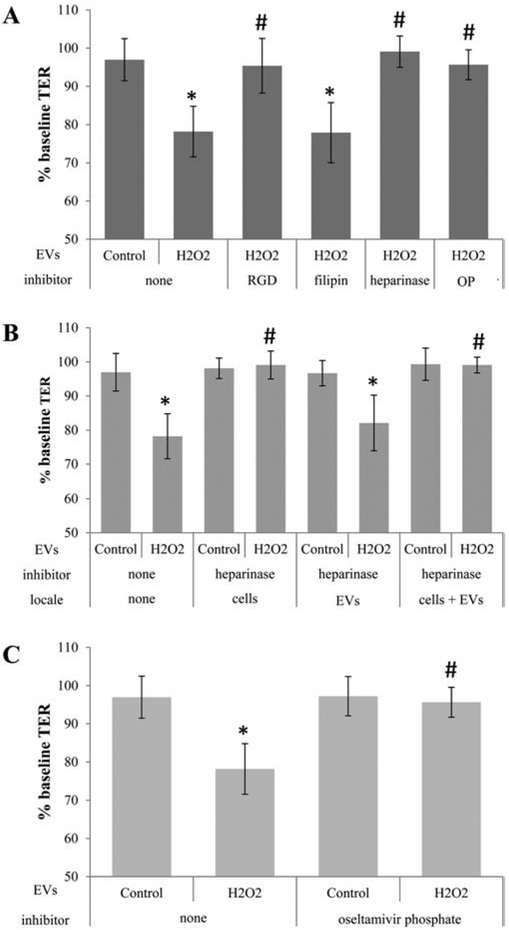
Transepithelial resistance (TER) assays are used to study bystander effects elicited by EV transfer as published, comparing TER measurements between 0 (baseline) and 4 hour time point (post treatment). A reduction in TER is taken as successful uptake of EVs by recipient cells [3]. Tables are arranged as follows indicating the type of EV (stress/H2O2 or control), the inhibitor used, and the location to which the inhibitor is added (recipient cell or EV), if location varies within a graph. (A) EVs from ARPE-19 cell monolayers (donor cells) of control or stressed (H2O2) were transferred directly to the apical side of naïve monolayers (recipient cells). EVs from stressed cells reduced TER. Pretreatment of recipient cells with RGD peptide (integrin receptor inhibitor), heparinase (removes surface proteoglycans) or oseltamivir phosphate (OP; inhibits neuraminidase activity) prevented TER reduction, whereas filipin (inhibits lipid-based interaction) did not. (B) Surface proteoglycans might play a role in binding of EVs to the recipient cell surface. Proteoglycans can be on the EV or the cell membrane of the recipient cell. Incubation of either cells, EVs or both with heparinase confirmed the importance of proteoglycans on the cell membranes of the recipient cells. (C) Transfer assays were repeated on EVs isolated from control or stressed donor ARPE-19 cells and preincubated with OP, which revealed the lack of an effect of OP added to EVs on TER. Bar graphs represent mean ±SEM. Significance was obtained using a posthoc ANOVA with Bonferroni correction. * indicates difference of indicated TER values with those elicited by control EVs; # indicates difference of indicated TER values with those elicited by stress (H2O2) EVs.
