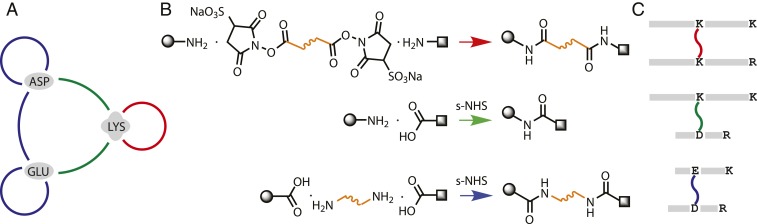
Fig. 1.
Overview of cross-linking chemistry and search/FDR strategy. (A) Targeting 3 of the most abundant residues—Lysine (LYS), Aspartate (ASP), and Glutamate (GLU)—on protein surfaces and all 6 possible cross-links among them with 3 reactions. (B) Bifunctional NHS-ester—the most common type of reagent—cross-links primary amines (red). EDC/sNHS chemistry is used to link carboxyl groups to primary amines, resulting in 0-length cross-links (green). Addition of a diamine linker to the EDC/sNHS reaction (E-EDC/sNHS) results in cross-links between carboxyl groups (blue). (C) Following tryptic digestion, the 3 cross-link types lead to peptides that theoretically should be 4, 3, and 2 times the size of a typical tryptic peptide, due to missed cleavage at the cross-link site.