Fig. 4.
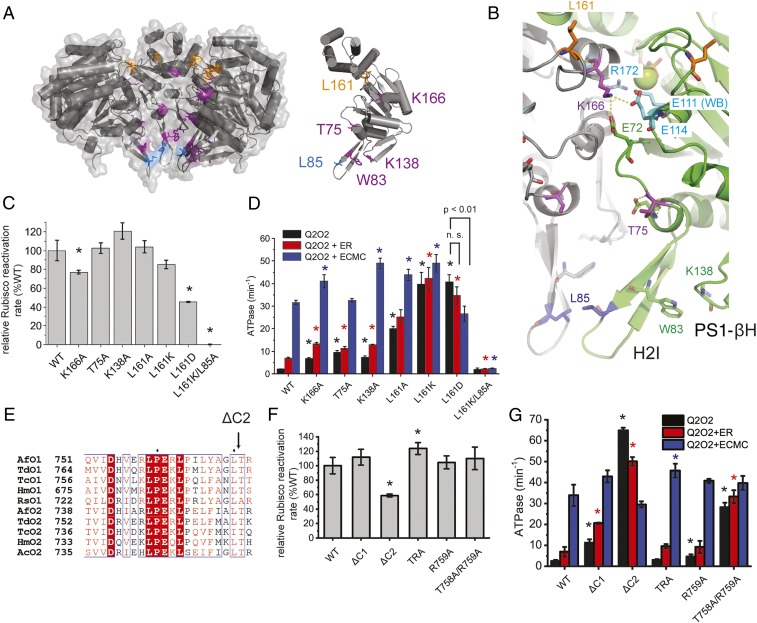
Residues involved in sensing or signaling the presence of inhibited Rubisco. (A) Visualization of CbbQ residues involved in substrate signaling in the hexamer (Left) or single subunit (Right) as in Fig. 3A. (B) Ribbon representation of adjacent subunits (green and gray) showing key CbbQ signaling residues in stick representation. L161 is located on the β4-α4 loop near the rim of the hexamer. Another residue on the same loop, K166, can form a salt bridge with E72 and E114. Mg2+ is shown as green sphere. (C) Relative Rubisco activase activities of CbbQ signaling mutants as described in Fig. 3C. (D) ATPase activity was measured in the absence and presence of inhibited Rubisco as described in Fig. 3D. n.s., not significant. (E) The length of CbbO C termini is highly conserved. The CbbO sequence alignment shown is that reported in ref. 15. Rca (F) and ATPase (G) activity assays of Q2O2 variants harboring mutations at the CbbO C terminus. Error bars indicate the mean and SD of at least 3 technical replicates. *P < Bonferroni-corrected α (C and D: 0.05/7; F and G: 0.05/5), Welch’s t test compared to WT values.