Significance
Human activities have played an important role in driving biodiversity loss throughout history, but the nature of these dynamics remains unclear. Importantly, the role of cultural evolution is mostly ignored, despite strong societal changes over time worldwide. Here, we show that megafauna range contractions across China in the last 2 millennia have been dominated by the spread of farming and agricultural intensification, often associated with expansion of the Han culture, with little or no direct importance of climate change. Our findings demonstrate that cultural evolution is important for shaping the assembly of contemporary communities from historical regional species pools and has long overshadowed climate change in driving even broadscale biodiversity patterns.
Keywords: extinction, cultural evolution, human migration, agricultural intensification, biodiversity conservation
Abstract
Human activities currently play a dominant role in shaping and eroding Earth’s biodiversity, but the historical dynamics leading to this situation are poorly understood and contentious. Importantly, these dynamics are often studied and discussed without an emphasis on cultural evolution, despite its potential importance for past and present biodiversity dynamics. Here, we investigate whether cultural filtering, defined as the impact of cultural evolution on species presence, has driven the range dynamics of five historically widespread megafauna taxa (Asiatic elephant, rhinoceroses, tiger, Asiatic black bear, and brown bear) across China over the past 2 millennia. Data on megafauna and sociocultural history were compiled from Chinese administrative records. While faunal dynamics in China are often linked to climate change at these time scales, our results reveal cultural filtering as the dominant driver of range contractions in all five taxa. This finding suggests that the millennia-long spread of agricultural land and agricultural intensification, often accompanied by expansion of the Han culture, has been responsible for the extirpation of these megafauna species from much of China. Our results suggest that cultural filtering is important for understanding society’s role in the assembly of contemporary communities from historical regional species pools. Our study provides direct evidence that cultural evolution since ancient times has overshadowed climate change in shaping broadscale megafauna biodiversity patterns, reflecting the strong and increasing importance of sociocultural processes in the biosphere.
The loss of biodiversity is one of the most worrying ecological consequences of Homo sapiens’ widespread activities, not only in the contemporary human-dominated biosphere with its strong extinction and extirpation trends (1), but also further back into the Holocene and the Late Pleistocene (2, 3), with climate change often argued as being a competing driver in both the present (4) and the past (5). According to the species pool model for community assembly across scales (6–8) (SI Appendix, Fig. S1A), the loss of species in a given area can be understood by considering dispersal, environmental, and biotic filters. However, it is increasingly clear that we have to elucidate humans’ role in driving species range dynamics at multiple spatiotemporal scales (2, 9–11).
One important causal process—cultural evolution—has been highlighted to explain H. sapiens’ unique ecological success (12, 13), with culture defined as information acquired through social learning that affects individual behavior (Methods). The adaptive and cumulative evolutionary process of socially learned culture has enabled humans to thrive in, shape, and coevolve with a wide range of ecological conditions via context-specific ecosystem-engineering behavior and strategies for subsistence, leading to sociocultural activities scaling up to the eventual emergence of H. sapiens as a force of global transformation with enduring ecological consequences (14). In this sense, ecological theories that do not consider anthropogenic impacts driven by cultural evolution are unlikely to explain and predict ecological patterns and processes in a biosphere increasingly shaped by human-mediated forces.
There are a growing number of studies that investigate associations between current ecological patterns and past human activities (2, 15), and it is sensible to recognize local landscapes as legacies of long-term culture–nature interactions. Nevertheless, human-related ecological dynamics at larger spatial scales are usually discussed in the context of generalized anthropogenic impacts, without an emphasis on the underlying cultural evolution (16–19), with a few exceptions from other related fields (e.g., refs. 20–23).
To better understand the role of cultural evolution in ecology, empirical studies are required to provide detailed information on the unfolding of culture–nature interactions across broad geographical extents. Although China’s long history and continuous, well-preserved written records (17–19, 22, 24), as well as accumulating archeological findings (25–27), provide a unique opportunity to test the role of cultural evolution in shaping broadscale ecological patterns across millennia, relevant quantitative research drawing on these historical data sources is still lacking.
Here, we first extend the classic species pool model by adding a cultural filter component to represent the impact of cultural evolution on the assembly of local communities from regional species pools (SI Appendix, Fig. S1 B and C). Furthermore, we investigate the quantitative effects of cultural filtering by using data (SI Appendix, Table S1) on the historical dynamics of the climate, human populations, agricultural intensification, cultural expansion, and the distributions of five megafauna taxa across eastern China (SI Appendix, Fig. S2) over the past 2 millennia. During this period, China has experienced a rapidly growing human population, the expansion and intensification of agriculture, and the spread of the Han culture, as well as climatic fluctuations (22). Our specific study questions were as follows: Has cultural filtering affected megafauna range dynamics across China over this time span, and in what manner has this occurred? What is the relative importance of climate change and cultural evolution in driving these range dynamics?
Results
Historical Range Dynamics in Five Megafauna Taxa.
We compiled historical records at the prefectural level (∼100 × 100 km) on each taxon’s presence and mapped the distribution dynamics of the taxa over time. In total, 709 records for the Asiatic elephant (Elephas maximus) in 410 localities from 6000 BCE (before the Common Era) to 1938 CE (Common Era); 1,277 records for rhinoceroses (Rhinoceros/Dicerorhinus spp.) in 614 localities from 702 BCE to 1962 CE; 3,822 records for the tiger (Panthera tigris) in 1,521 localities from 4376 BCE to 1976 CE; 1,232 records for the Asiatic black bear (Ursus thibetanus) in 610 localities from 210 BCE to 1982 CE; and 324 records for the brown bear (Ursus arctos) in 186 localities from 416 BCE to 1990 CE were compiled in our study.
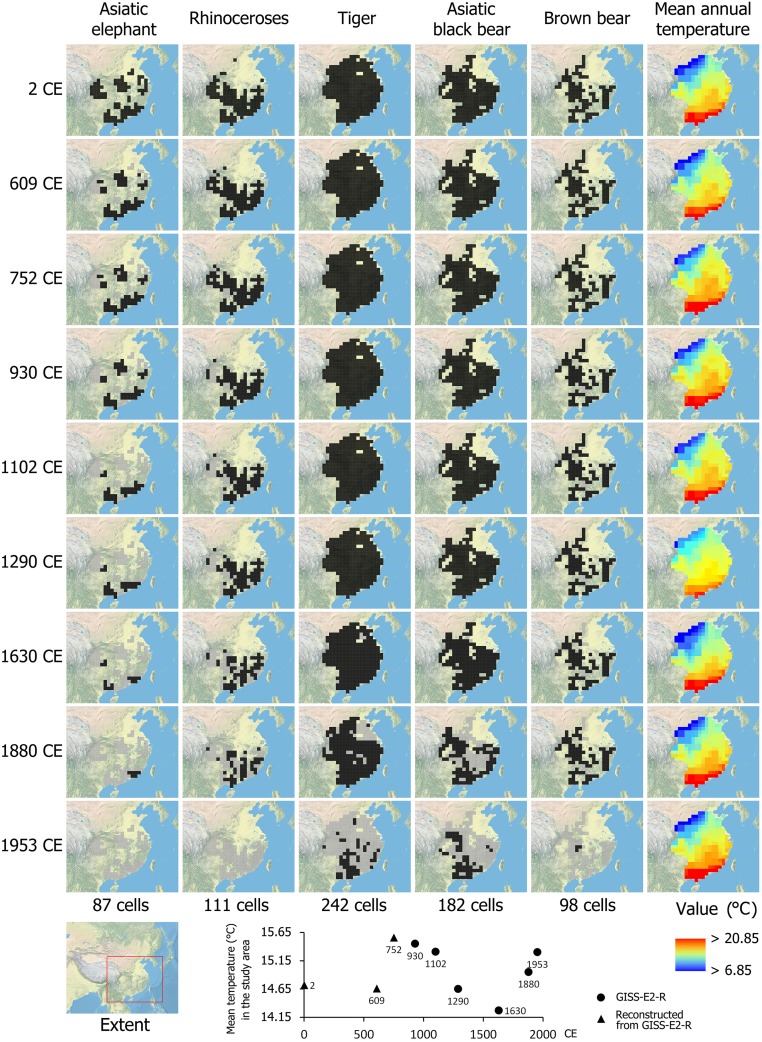
All five megafauna taxa were widely distributed across eastern China 2,000 y ago, with records in both the lowlands and the uplands (Fig. 1). Subsequently, the Asiatic elephant and rhinoceroses experienced strong, progressive range contractions over time, finally disappearing from the study area by the mid-20th century (with a small population of elephants still persisting in Southwest China just outside the study area). In contrast, the distributions of the tiger, Asiatic black bear, and brown bear remained almost stable until their recent rapid range losses across the late 19th to the mid-20th century. The overall spatial gradient of temperature in eastern China has remained largely stable over the past 2 millennia (Fig. 1), with only minor temporal trend shifts and local variations, inconsistent with the strong range dynamics of these megafauna species.
Fig. 1.
Distribution dynamics of five megafauna taxa and spatial patterns of mean annual temperature in eastern China over the past 2 millennia. For each taxon, the cells colored black on the map are locations where the taxon was present by the given time, while the cells colored gray on the map are locations where the taxon was no longer recorded by the given time.
Sociocultural Development in Ancient China.
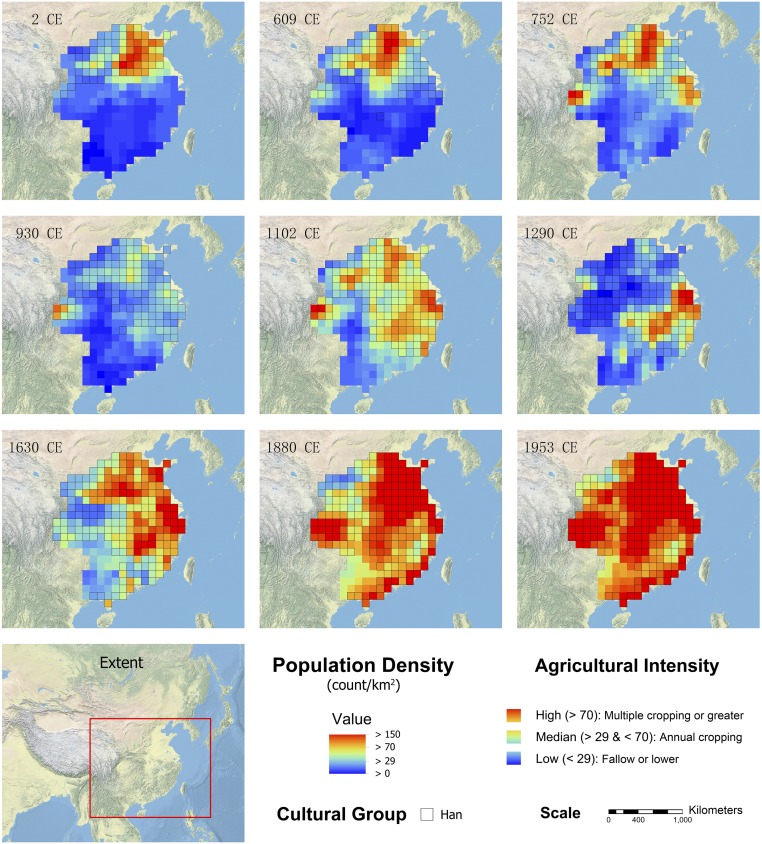
We reconstructed maps (Fig. 2) for variables of human population density, agricultural intensification, and major cultural groups (Han vs. non-Han; see SI Appendix, Supplementary text for explanation) to represent factors that may drive cultural filtering. Cultural filtering can function via pathways of between- and within-society evolution, as well as culturally learned behavior of individuals. In our multiple-regression analysis (Methods), human population density represents the individual behavior pathway through land alteration for farming, while agricultural intensity represents the within-society evolutionary pathway through sociocultural changes associated with increasing societal complexity. Finally, the major cultural groups capture the between-society evolutionary pathway through intersocietal interactions. Human population density is expected to be negatively related to megafauna presence, given the associated conversion of wildlife habitat to agricultural land (28, 29). The effects of agricultural intensification are less straightforward, given the simultaneous higher land-use efficiency and greater societal demands for ecosystem services (30), but an overall negative relationship with megafauna distributions is expected (31). The effects of the Han culture are expected to be more noticeable than those of non-Han cultures, as the classic Han culture has a more aggressive attitude toward nature than non-Han cultures do (22, 24).
Fig. 2.
Spatial patterns of human population density, agricultural intensity, and the Han culture in eastern China over the past 2 millennia. These three variables represent factors that may drive cultural filtering (Methods) via pathways of individual behavior, within-, and between-society evolution, respectively. Population density 29 and 70 are used as threshold values to classify the color ramp into three levels of agricultural intensity (see SI Appendix, Supplementary text for details).
Two thousand years ago, dense human populations supported by intensive agricultural practices with the Han culture were mainly clustered on the North China Plain (Fig. 2). Eleven centuries later, agricultural intensification and the Han culture spread southward, and multiple hotspots of dense human populations emerged in southern China as well as in the north. As a result of subsequent demographic and societal dynamics, most lowland areas and some upland areas became densely populated before the 20th century, concurrent with the further spread of the Han culture. Most arable land in modern China has been under highly intensive agricultural use since the mid-1950s.
Drivers of Megafauna Range Contractions.
We applied fixed-effects logit models to assess the effects of climatic and cultural filtering on species presence (Methods). The statistical results show that variables representing sociocultural processes explained the range contractions of all five taxa and were the only variables with explanatory power for four taxa, namely, the Asiatic elephant, rhinoceroses, the Asiatic black bear, and the brown bear (Table 1). For the tiger, temperature was also important. At the cultural macroevolutionary level, only the Asiatic elephant had a simple negative response to the Han culture variable, which also had estimated effects on the other taxa through interaction terms. The effects of agricultural intensification were 2-fold. At the cultural microevolutionary level, indirect effects associated with agricultural intensification (i.e., the coefficients of the annual cropping and multiple cropping terms in Table 1) had consistently negative effects on all five megafauna taxa. Complementing this effect, the negative effects of agricultural land expansion at the individual behavior level (i.e., the negative coefficients of the population density term in Table 1) decreased consistently with agricultural intensification (i.e., the positive coefficients of the population-density-related interaction terms in Table 1), but did not become positive. Variables representing sociocultural processes were always among the identified predictors of the range dynamics of all five megafauna taxa (SI Appendix, Tables S3–S7), and models that accounted for cultural filtering performed better in terms of both the Bayesian information criterion (BIC) and the Akaike information criterion than those that did not (Table 1; see also SI Appendix, Tables S3–S7).
Table 1.
Coefficient estimates for the fixed-effects logit models describing the local (scale of 100 × 100 km) presence of five megafauna taxa over the past 2 millennia across eastern China selected according to the BIC
| Megafauna taxa | The lowest BIC model(s) | |||||||||||
| MAT | PD | CG | AC | MC | PD×CG | PD×AC | PD×MC | AC×CG | PD×AC×CG | PD×MC×CG | BIC | |
| Asiatic elephant | — | −0.054 | −1.65 | — | — | 0.012 | 0.003 | — | — | — | 0.022 | 1,039.65 |
| Rhinoceroses | — | −0.076 | — | — | −3.324 | — | — | 0.041 | — | 0.0013 | — | 1,311.62 |
| — | −0.071 | — | — | −3.370 | — | — | 0.037 | −0.388 | — | — | 1,311.15 | |
| Tiger | −1.070 | −0.027 | —* | −1.286 | −1.843 | 0.005 | — | — | — | — | — | 2,412.21 |
| −1.093 | −0.022 | —* | −1.567 | −1.909 | — | — | — | — | 0.0059 | — | 2,411.61 | |
| Asiatic black bear | — | −0.068 | — | — | −1.56 | — | — | 0.039 | — | 0.018 | — | 1,802.63 |
| Brown bear | — | −0.100 | — | — | −2.927 | — | — | 0.070 | — | 0.019 | — | 1,031.28 |
| — | −0.108 | — | — | −3.332 | — | — | 0.078 | −2.472 | 0.059 | 1,031.91 | ||
See SI Appendix for model selection criteria. Variables include mean annual temperature (MAT), human population density (PD), cultural group (CG), annual cropping (AC), and multiple cropping (MC). The results of significance tests are also presented for reference: Numbers in bold are significant at the 0.01 level, and numbers in italics are significant at the 0.05 level. There are two lowest BIC models for some taxa. See SI Appendix, Table S2 for full modeling results.
Note that including the CG term directly in the models for the tiger always led to fitting failure.
Discussion
Interpreting the Effects of Cultural Filtering.
Our results clearly show that the multiple filtering effects of sociocultural processes have been the main drivers of the distributions of the studied megafauna taxa over the past 2 millennia in eastern China (Table 1), a period during which agriculture has been the foundation for both individual livelihoods and overall society (32). First, at the individual behavior level, land-use expansion for agriculture, which is culturally learned, was confirmed to consistently reduce the ranges of all five megafauna taxa at the large spatiotemporal scale studied, consistent with our expectation that habitat loss due to agriculture is one of the major threats to mammal diversity. Human-induced habitat loss is a long-established major driver of regional and global megafauna declines throughout the Holocene and in the Anthropocene (19, 33, 34). Similarly, many freshwater species have been extirpated due to land reclamation of wetlands in the study region (35).
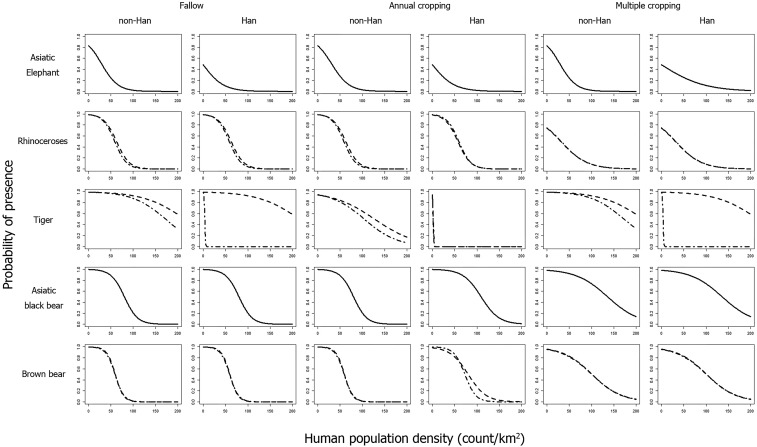
Second, at the level of cultural microevolution, increasing societal complexity and technical productivity, partly reflected by agricultural intensification, were estimated to have had mixed effects on megafauna distributions. On the one hand, higher societal complexity associated with human population growth (36) usually places extra demands on natural resources, including various ecosystem provisioning services, resulting in pressure on the local presence of some taxa (30), in line with our findings (in Fig. 3, compare the shapes of the curves for a particular taxon among the three agricultural intensity levels). On the other hand, increased cropland productivity due to intensive agricultural technologies was also confirmed to reduce the per-unit human population-growth effects on the five megafauna taxa, suggesting that agricultural intensification may partially alleviate per-capita ecological impacts (Fig. 3; the probability of megafauna presence at high human-population densities is greater under annual or multiple cropping than for fallow-based agriculture).
Fig. 3.
Probability of the local (scale of 100 × 100 km) presence of five megafauna taxa estimated with the models in Table 1. Solid lines indicate taxa that have only a single lowest BIC model in Table 1, while point–dash and dashed lines indicate taxa that have two effectively equally lowest BIC models in Table 1. Modeling and plotting were implemented in R software (76) using the bife package (75).
However, the coefficients of the population-density term in Table 1 are always negative, even for annual or multiple cropping, indicating that agricultural intensification in Imperial China did not arrest agricultural land expansion into the habitats of the five taxa, inconsistent with the “land-sparing” hypothesis (28). Moreover, the finding shown in Table 1 that the coefficient of multiple cropping for each taxon is larger than that of annual cropping suggests that sociocultural changes associated with agricultural intensification can intensify the impacts on megafauna distributions at these broad scales. Therefore, cultural microevolution represented by agricultural intensification had overall negative filtering effects on the megafauna taxa in eastern China, representing transformation from biodiverse natural landscapes into productive croplands (19, 22). In ancient Egypt, millennia-long societal changes in response to aridification, with intensifying resource exploitation, were also associated with extirpations of many large-bodied mammal species (37). Importantly, in industrialized societies, urbanization and land-use intensification are major socioeconomic drivers of farmland abandonment (14, 38) and thus have the potential to provide both food security and nature recovery, including the return of locally extinct mammal species (39–41).
Third, simple, direct cultural filtering by Han vs. non-Han cultural groups was found only for the Asiatic elephant, although interactive effects of the Han culture with other drivers were found for some of the other taxa (Table 1). Notably, the tiger also shows distinct probability curves in response to Han vs. non-Han cultural groups (Fig. 3); however, this effect was not well estimated during the model fitting (SI Appendix, Supplementary text). Although the spread of the Han culture has resulted in intensified local land use for farming and reshaped natural landscapes (22, 42), no explicit evidence is available to suggest that the Asiatic elephant is particularly sensitive to the Han culture, as there are historical records showing that both Han and non-Han cultural groups hunted the Asiatic elephant for tusks or meat and as a nuisance species (24), with human–elephant conflicts also reported elsewhere around the world (43). However, it is important to note that agriculture has played a more important role in the Han culture than in non-Han cultures in China (32) and thus should be considered to have a greater influence on activities and factors shown to be detrimental to crop production. Hence, it is sensible to argue that conflicts between Han societies and wildlife are likely to have been stronger, especially those involving herbivores that damage crops, with the Asiatic elephant providing evidence for cultural macroevolutionary filtering (see narratives in ref. 24 for past conflicts between Han Chinese and the Asiatic elephant). Similar filtering effects on biodiversity can also be found in the Brazilian Amazon (44) and tropical northern Australia (45), where lands inhabited by indigenous, less agricultural people are subject to lower biodiversity losses than other inhabited lands.
Furthermore, as shown in Fig. 2, the spread of the Han culture was generally concurrent with agricultural intensification from low intensity to intermediate or high intensity, likely due to the focus of the Han culture on crop production. Hence, in Table 1, the two positive coefficients of the population-density–cultural group interaction (PD×CG) term for the Asiatic elephant and tiger and the two negative coefficients of the annual cropping–cultural group interaction (AC×CG) term for rhinoceroses and the brown bear are reflections of the cross-level link (the horizontal spread of microevolution associated with macroevolution) between the Han culture and intensive agricultural production: When the Han culture is present (CG = 1), intensive agriculture (e.g., annual cropping) becomes widespread and has the 2-fold effects on megafauna discussed above—the negative effects associated with agricultural intensification (reflected by the negative AC×CG term) and the alleviation of per-unit population growth effects on megafauna (reflected by the positive PD×CG term). Indeed, it is not uncommon for cultural or ethnic groups to spread at the expense of others, with newly arriving cultures reshaping previous culture–nature relationships and, by extension, nature (20, 21). For example, the global spread of H. sapiens, characterized by cultural evolution (13), has been argued to be the main driver of the late Quaternary megafauna extinction, reshaping continental megafauna communities that in some cases had coexisted with other hominins across long time spans (2). Changes in fire regimes and biotic interactions caused by European settlement in Australia have led to an extraordinary rate of mammal extinction, at least partly coupled to the loss of Aboriginal culture (46). Analogously, large carnivores and ungulates in North America have experienced notable range contractions since European settlement (16).
The Role of Climate Change.
Changes in temperature were unable to explain the range contractions of the megafauna taxa considered in this study (see SI Appendix for our interpretation of the negative coefficient of temperature for the tiger in Table 1), in contrast to previous work on mammal range contractions in China (e.g., ref. 17). There are at least two possible reasons for our results. First, the human-population data used here were derived from recent publications on the population history of China by historians who have meticulously interpreted and calibrated (Methods) raw historical human population records, rather than using these in uncalibrated fashion (cf. ref. 17). Due to the incompleteness of premodern censuses, the raw records generally underestimate the actual human population size for the period from 140 to 1776 CE (47)—particularly by a half or more for the Song Dynasty (980 to 1279 CE), the Ming Dynasty (1393 to 1644 CE), and the early Qing Dynasty (until 1776 CE) (Methods), i.e., during periods where major range contractions of the Asiatic elephant and rhinoceroses occurred (Fig. 1). The spatial noise resulting from such underestimates of human-population size is likely to transfer statistical importance from anthropogenic impacts to other factors, e.g., climate change. The other potential reason is that failing to account for sociocultural processes that are associated with demographic dynamics when the goal is to assess explanatory effects by means of regression modeling (cf. ref. 17) violates the fundamental assumption of exogeneity for regression models and can reduce the credibility of coefficient estimates and significance tests (48, 49). Finally, concerning the role of precipitation, no clear patterns have been identified in terms of the past precipitation changes in this region over the study period (50), inconsistent with the mostly southward contractions of the five megafauna taxa.
Importantly, although cultural filtering has been found to overshadow environmental filtering as a driver of megafauna distributions in this study, the latter should not necessarily be dismissed as having no importance for the overall distribution of these taxa. Notably, the absence of historical records for the Asiatic elephant and rhinoceroses in other parts of China (e.g., northeastern China) that are connected to our study area, but have a relatively colder climate, is consistent with broadscale climatic conditions delimiting the ecological boundaries of these megafauna taxa in this part of the study region. The tiger, a highly adaptive species that was distributed across all parts of China with differing macroclimate and topography, thrives only in forests, savannas, or wetlands (51), exemplifying the importance of habitat processes in the occurrence of the species across multiple biomes and biogeographical regions. At regional to global scales, climate has repeatedly been reported to have played an important role in driving changes in the distribution and abundance of megafauna across the late Quaternary (3, 5, 37, 52, 53), while likely not being the cause of the global megafauna extinctions in this period (2, 54). Moreover, in contrast to earlier times, when climate change was largely exogenous to human societies, now—in the Anthropocene (55)—human activities under strong sociocultural change and upscaling (14) are closely intertwined with global climate change as the main driver of these, with increasingly strong impacts on ecosystems and species distributions (56). Furthermore, now interactions between different filtering processes may cause increased uncertainties regarding biodiversity changes and conservation effectiveness (57).
Implications for Conservation.
The explicit consideration of cultural filtering in the classical species-pool model has major implications for better understanding species-pool changes caused by anthropogenic processes and thereby for biodiversity conservation strategies in a human-dominated world. The inclusion of cultural filtering highlights the active role of culture in ecological processes and consequences. This role is disregarded in the classical model, in which anthropogenic impacts actually driven by culture are usually decomposed into the three classical filters (dispersal, abiotic, and biotic conditions); thus, the consideration of culture may inspire questions such as how to modify cultural filtering in order to attain desired ecological consequences.
One example related to this question is the population decline of the South China tiger (Panthera tigris amoyensis), which was accelerated by “antipest” campaigns in the 1950s that targeted this subspecies (58). For comparison, another example is the strong reexpansion of large mammal species across Europe due to sociocultural changes, including the development of conservation policies and hunting regulations, agricultural land abandonment, and supportive public opinion, over the past several decades (59). Such cases provide evidence that sociocultural processes can be utilized to effectively filter out or bring back certain species over short time periods across large spatial extents. Therefore, it is reasonable to emphasize the potential of intentionally regulating society’s responses to target species, with proper consideration of sociocultural context and ecological uncertainties (60–62). However, cultural values, defined as principles that guide human behavior, are not easily changed (63). In addition to top-down policies, decision-making processes that incorporate local people’s perspectives on and experiences in living with wildlife are also indispensable to addressing repeated human–wildlife conflicts (64). Enriching our knowledge about how cultural filtering, as well as its interactions with other ecological processes, shapes biodiversity at multiple scales is an important future direction for research on biodiversity conservation and ecosystem restoration in an anthropogenic biosphere (43, 64, 65), especially as the Anthropocene advances, with a strong and increasing human influence on the Earth system driven by ever-evolving culture (14, 66).
Conclusion
Our study drawing on historical data that span the past 2 millennia quantitatively shows that sociocultural processes have caused the extensive range contractions of five megafauna taxa formerly widely distributed across eastern China. Our results provide direct support for the explicit consideration of cultural filtering in the classical species-pool model, even at the broad scales at which major natural forces, such as climate, are often believed to be the dominant drivers of ecological change. The finding that anthropogenic activities driven by ever-evolving culture have overshadowed other forces in shaping species distributions for millennia highlights the need to guide sociocultural change in response to biodiversity challenges in the Anthropocene, with help from ecological knowledge and theories on sociocultural processes.
Methods
Species-Pool Model with Cultural Filtering.
The contemporary biosphere is strongly and increasingly transformed by anthropogenic forces. In this situation, the classical species-pool model (6) is inadequate for explaining and predicting community dynamics, as it may no longer be realistic to assume that regional species pools do not change at ecological timescales (7). Moreover, the species-pool model usually interprets human impacts as biotic filtering (e.g., via hunting, extirpation, or introduction) and/or environmental filtering (e.g., via landscape transformation) (67), failing to adequately consider how modern humans (H. sapiens) dramatically differ among locations in terms of their interactions and coexistence with other species, as well as humans’ wide-ranging ability to not only adapt to but also modify environmental conditions (13, 14).
Cultural filtering is defined as the ecological impacts of cultural evolution in terms of community assembly. Species-pool models that include cultural filtering, in addition to dispersal filtering, environmental filtering, and biotic filtering (SI Appendix, Fig. S1B), can address the potential inappropriateness of assuming static regional species pools in an anthropogenic biosphere increasingly shaped by sociocultural activities that occur at unprecedented scales and rates, where these activities are essentially driven by cultural evolution. Broadly speaking, as culture is defined as information acquired through social learning and affects how individuals behave (13, 68), any ecological consequences of human behavior (e.g., hunting, fire use, agricultural land use, and industrial ecosystem engineering) can be treated as outcomes of different cultural filters. Therefore, in this case, the loss of biodiversity resulting from human activities is not considered from the perspective of biotic (e.g., competition) or environmental (e.g., habitat loss) filtering, but explained by cultural filtering. Why and how culture evolves are not explained by such cultural species pool models, and thus remain exogenous to these models; however, human-induced changes in species pools and communities, which are often as widespread and rapid as those caused by climate change (14), thus invalidating the standard assumption that regional species pools are relatively stable (7), are now incorporated in these models.
Cultural evolution, which is analogous to biological evolution, consists of two levels: microevolution within a social population or group and macroevolution across social populations (69). Microevolution involves social, cultural, and technical dynamics across generations within a society, while variation in sociocultural traits (e.g., languages and customs) across societies and ethnic entities is a reflection of macroevolution. In this sense, cultural filtering can function via pathways of both macroevolution and microevolution, as well as individual behavior shaped by culture (cf. ref. 14). Considering cultural filtering in agricultural societies as an example, the individual behavior pathway of filtering involves land alteration for farming by individuals who obtain the requisite knowledge socially. The microevolutionary pathway involves sociocultural changes associated with factors such as crop productivity, demographic dynamics, agricultural technologies, and urbanization, while the macroevolution pathway involves competition and the interactions of power, values, and ethnicity across agricultural societies. All three pathways function as filters for local community assembly from a regional species pool in an agricultural setting.
Spatiotemporal Data.
To test for empirical evidence of the effects of cultural filtering on species pools at a large spatiotemporal scale, we collected data on the distribution dynamics of five megafauna taxa, the human population, and related sociocultural characteristics (i.e., agricultural intensification and the expansion of the Han culture) in China over the past 2 millennia (Datasets S1–S5; see SI Appendix for details). As the boundaries of ancient China and the geographical extent of these historical data varied over time, we restricted our study to the area referred to as eastern China (SI Appendix, Fig. S2), while fully recognizing that the area consists of parts belonging to East, Central, North, South, and Southwest China.
The five studied megafauna taxa were the Asiatic elephant (E. maximus), Asian rhinoceroses (Rhinoceros sondaicus, Rhinoceros unicornis, and Dicerorhinus sumatrensis), the tiger (P. tigris), the Asiatic black bear (U. thibetanus), and the brown bear (U. arctos). The presence of R. sondaicus and D. sumatrensis in ancient China has been confirmed by zooarchaeological evidence, while only written descriptions are currently available for R. unicornis (ref. 70; see also SI Appendix, Supplementary text). We analyzed the three rhinoceros species as a single taxon group due to insufficient information in the original historical materials for species- or genus-level identification (70), as done in several recent studies using the distribution data for rhinoceroses in ancient China (17, 19, 71). For each taxon, the most recent record in the dataset for a prefecture was interpreted as the taxon’s last occurrence in that prefecture, with the underlying assumption that the taxon was constantly present there until the last occurrence (18).
As the human population count for a given geographical unit equals the population density times the area, agricultural population growth can be attributed to either agricultural land expansion (i.e., increased agricultural land use) or agricultural intensification (i.e., increased population density). When agricultural use within a geographical unit (e.g., a prefecture or grid cell) is known and fixed, increases in the unit’s population density are attributed to agricultural land expansion; on the other hand, as agricultural intensification and sociocultural complexity increases are closely associated with population growth, it would only be meaningful to assess the indirect partial effects instead of direct partial effects of agricultural intensification when holding the population density fixed (cf. ref. 48); such effects include labor division, social stratification, natural-resource demands, and so on. Therefore, information regarding the intensity of agricultural use is additionally needed to distinguish the ecological impacts caused by agricultural land expansion on megafauna mammal distributions from those caused by agricultural intensification (see SI Appendix for details).
The years 2 CE, 609 CE, 752 CE, 930 CE, 1102 CE, 1290 CE, 1630 CE, 1880 CE, and 1953 CE were chosen to capture the past dynamics of demography, sociocultural development, and climate change in this study (32, 72, 73). The human population geography for 930 CE was assumed to be the same as that for 980 CE, the first year following the Five Dynasties and Ten Kingdoms Period (from 907 to 979 CE), in which frequent political upheavals resulted in the collection of very little reliable information that would allow researchers to reconstruct nationwide population counts and distributions (32, 72). The reconstruction map for each of these years was converted to 100- × 100-km grid cells. Every grid cell contained data on the presence/absence of megafauna taxa, the human population density, the level of agricultural intensity, the presence/absence of the Han culture, and the mean annual temperature (SI Appendix, Table S1).
Statistical Modeling and Analysis.
Our study focused on assessing the effects of sociocultural processes on the binary responses of megafauna mammal distributions over time. To this end, we applied fixed-effects logit models (also known as conditional logistic models) (74) to the spatiotemporal gridded data described above. The mathematical form of the model is as follows:
| [1] |
where i (= 1, 2, …, N) denotes a grid cell; t (= 1, 2, …, T) represents a time point; f is the logistic function f(z) = exp(z)/[1 + exp(z)]; xit is a row vector of K regressors, (x1it, x2it, …, xKit); Pr(yit = 1|xit) denotes the probability that yit = 1 (i.e., the taxon is present in location i at time t) conditional on xit; β is a column vector of coefficients, (β1, β2, …, βK); xitβ represents β1x1it + β2x2it + … + βKxkit; and αi represents time-invariant factors (referred to as fixed effects) for grid cell i.
More specifically, the explanatory variables were mean annual near-surface air temperature (x1; continuous), human population density (x2; continuous), level of agricultural intensity (x3; categorical), and cultural group (x4; categorical). As topographical conditions (e.g., slope and elevation) at the 100- × 100-km scale were assumed to be constant over the past 2 millennia, their effects were accounted for by the fixed-effects term α. Although it may be argued that the expansion of agricultural land use into highlands usually displaces large mammals to more sloped areas, resulting in a pattern that mammals appear to prefer remote habitats, it is important to note that, in this case, land-use change for agriculture rather than changes in topography reshapes mammal distributions across different landscapes. In other words, when there are no changes in other variables, the partial effects of topography on mammal distributions will remain the same.
We specified the following fixed-effects logit model:
| [2] |
| [3] |
| [4] |
| [5] |
where Logist(0, 1) denotes the standard logistic distribution; Xi is a matrix of regressor vectors xit (t = 1, 2, …, T); and αi is routinely assumed to be correlated with Xi in order to prevent the endogeneity problem in regression analysis of longitudinal data (48, 75). The coefficients β0 and β1 measure the response of a taxon to climate change; β2 measures the response of a taxon to population growth that can be attributed only to land conversion for agriculture when other variables are held fixed; and β3 measures the indirect effects of agricultural intensification on megafauna mammal distributions, as the close link between agricultural intensity and population density prevents the interpretation of β3 as the direct effect of agricultural intensification while population density is held fixed (cf. ref. 48). Furthermore, as β2 may change with intensity level, we also considered the interaction term x2it × x3it to capture changes (i.e., β5) in β2 due to agricultural intensification. β4 measures the response of a taxon to cultural group, while changes (i.e., β6) in β2 associated with cultural group are captured by the interaction term x2it × x4it. In addition, we considered the interactive effects between agricultural intensity level (i.e., x3it) and cultural group (i.e., x4it) by the two terms x3it × x4it and x2it × x3it × x4it, as changes in the two variables can be mutually dependent: Adoption of intensive agricultural technologies may be concurrent with cultural group change.
Data and R scripts for modeling are in SI Appendix. Data for reproducing the maps in this study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
We thank Erle Ellis for inspiration during a workshop in Zaragoza. S.N.T. was supported by China Scholarship Council Grant 201406190179, China Postdoctoral Science Foundation Grant 2019M660110, and the Postdoctoral International Exchange Program of the China Postdoctoral Council. J.-C.S. was supported by European Research Council Grant ERC-2012-StG-310886-HISTFUNC and also considers this work a contribution to his VILLUM Investigator project (VILLUM FONDEN Grant 16549) and his Carlsberg Foundation Semper Ardens project MegaPast2Future (Grant CF16-0005). C.X. was supported by National Natural Science Foundation of China Grant 31770512.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909896116/-/DCSupplemental.
References
- 1.Ceballos G., et al. , Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandom C., Faurby S., Sandel B., Svenning J.-C., Global Late Quaternary megafauna extinctions linked to humans, not climate change. Proc. Biol. Sci. 281, 20141574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett L. J., et al. , Robustness despite uncertainty: Regional climate data reveal the dominant role of humans in explaining global extinctions of Late Quaternary megafauna. Ecography 39, 152–161 (2016). [Google Scholar]
- 4.Mantyka-Pringle C. S., Martin T. G., Rhodes J. R., Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Change Biol. 18, 1239–1252 (2011). [Google Scholar]
- 5.Lorenzen E. D., et al. , Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pärtel M., Zobel M., Zobel K., van der Maarel E., The species pool and its relation to species richness: Evidence from Estonian plant communities. Oikos 75, 111–117 (1996). [Google Scholar]
- 7.Cornell H. V., Harrison S. P., What are species pools and when are they important? Annu. Rev. Ecol. Evol. Syst. 45, 45–67 (2014). [Google Scholar]
- 8.Kraft N. J. B., et al. , Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 29, 592–599 (2015). [Google Scholar]
- 9.Rapacciuolo G., et al. , The signature of human pressure history on the biogeography of body mass in tetrapods. Glob. Ecol. Biogeogr. 26, 1022–1034 (2017). [Google Scholar]
- 10.McGill B. J., Dornelas M., Gotelli N. J., Magurran A. E., Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 30, 104–113 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Birks H. J. B., Felde V. A., Seddon A. W. R., Biodiversity trends within the Holocene. Holocene 26, 994–1001 (2016). [Google Scholar]
- 12.Boyd R., Richerson P. J., Henrich J., The cultural niche: Why social learning is essential for human adaptation. Proc. Natl. Acad. Sci. U.S.A. 108 (suppl. 2), 10918–10925 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrich J., The Secret of Our Success: How Culture is Driving Human Evolution, Domesticating Our Species, and Making Us Smarter (Princeton University Press, Princeton, NJ, 2015). [Google Scholar]
- 14.Ellis E. C., Ecology in an anthropogenic biosphere. Ecol. Monogr. 85, 287–331 (2015). [Google Scholar]
- 15.Boivin N. L., et al. , Ecological consequences of human niche construction: Examining long-term anthropogenic shaping of global species distributions. Proc. Natl. Acad. Sci. U.S.A. 113, 6388–6396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laliberte A. S., Ripple W. J., Range contractions of North American carnivores and ungulates. Bioscience 54, 123–138 (2004). [Google Scholar]
- 17.Li X., et al. , Human impact and climate cooling caused range contraction of large mammals in China over the past two millennia. Ecography 38, 74–82 (2015). [Google Scholar]
- 18.Turvey S. T., Crees J. J., Di Fonzo M. M. I., Historical data as a baseline for conservation: Reconstructing long-term faunal extinction dynamics in late imperial-modern China. Proc. Biol. Sci. 282, 20151299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turvey S. T., Crees J. J., Li Z., Bielby J., Yuan J., Long-term archives reveal shifting extinction selectivity in China’s postglacial mammal fauna. Proc. Biol. Sci. 284, 20171979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosby A., Ecological Imperialism: The Biological Expansion of Europe, 900-1900 (Cambridge University Press, Cambridge, UK, 1986). [Google Scholar]
- 21.Diamond J., Evolution, consequences and future of plant and animal domestication. Nature 418, 700–707 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Marks R. B., China: Its Environment and History (Rowman & Littlefield Publishers, Lanham, MD, 2011). [Google Scholar]
- 23.Hoag C., Svenning J.-C., African environmental change from the Pleistocene to the Anthropocene. Annu. Rev. Environ. Resour. 42, 27–54 (2017). [Google Scholar]
- 24.Elvin M., The Retreat of the Elephants: An Environmental History of China (Yale University Press, New Haven, CT, 2004). [Google Scholar]
- 25.Liu F., Feng Z., A dramatic climatic transition at ∼4000 cal. yr BP and its cultural responses in Chinese cultural domains. Holocene 22, 1181–1197 (2012). [Google Scholar]
- 26.Hosner D., Wagner M., Tarasov P. E., Chen X., Leipe C., Spatiotemporal distribution patterns of archaeological sites in China during the Neolithic and Bronze Age: An overview. Holocene 26, 1576–1593 (2016). [Google Scholar]
- 27.Zhao L., et al. , Holocene vegetation dynamics in response to climate change and human activities derived from pollen and charcoal records from southeastern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 485, 644–660 (2017). [Google Scholar]
- 28.Green R. E., Cornell S. J., Scharlemann J. P. W., Balmford A., Farming and the fate of wild nature. Science 307, 550–555 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Phalan B., Onial M., Balmford A., Green R. E., Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science 333, 1289–1291 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Bommarco R., Kleijn D., Potts S. G., Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen L. V., et al. , Social-ecological outcomes of agricultural intensification. Nature Sustainability 1, 275–282 (2018). [Google Scholar]
- 32.von Glahn R., The Economic History of China: From Antiquity to the Nineteenth Century (Cambridge University Press, Cambridge, UK, 2016). [Google Scholar]
- 33.Dirzo R., et al. , Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Young H. S., et al. , Patterns, causes, and consequences of Anthropocene defaunation. Annu. Rev. Ecol. Evol. Syst. 47, 333–358 (2016). [Google Scholar]
- 35.Wang L., Aquatic environments and fishery production in Middle Imperial North China. J. Chin. Hist. Geogr. 14, 44–55 (1999). (in Chinese). [Google Scholar]
- 36.Turchin P., et al. , Quantitative historical analysis uncovers a single dimension of complexity that structures global variation in human social organization. Proc. Natl. Acad. Sci. U.S.A. 115, E144–E151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeakel J. D., et al. , Collapse of an ecological network in Ancient Egypt. Proc. Natl. Acad. Sci. U.S.A. 111, 14472–14477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lasanta T., et al. , Space-time process and drives of land abandonment in Europe. Catena 149, 810–823 (2017). [Google Scholar]
- 39.Navarro L. M., Pereira H. M., Rewilding abandoned landscapes in Europe. Ecosystems 15, 900–912 (2012). [Google Scholar]
- 40.Queiroz C., Beilin R., Folke C., Lindborg R., Farmland abandonment: Threat or opportunity for biodiversity conservation? A global review. Front. Ecol. Environ. 12, 288–296 (2014). [Google Scholar]
- 41.Li S., Li X., Global understanding of farmland abandonment: A review and prospects. J. Geogr. Sci. 27, 1123–1150 (2017). [Google Scholar]
- 42.Chen J., Opening up of the Eastern Region land during the Shang and Zhou dynasties and southward migration of elephants. Hist. Res. 56, 4–18 (2016). (in Chinese). [Google Scholar]
- 43.Barua M., Bhagwat S. A., Jadhav S., The hidden dimensions of human-wildlife conflict: Health impacts, opportunity and transaction costs. Biol. Conserv. 157, 309–316 (2013). [Google Scholar]
- 44.Nepstad D., et al. , Inhibition of Amazon deforestation and fire by parks and indigenous lands. Conserv. Biol. 20, 65–73 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Yibarbuk D., et al. , Fire ecology and aboriginal land management in central arnhem land, northern Australia: A tradition of ecosystem management. J. Biogeogr. 28, 325–343 (2001). [Google Scholar]
- 46.Woinarski J. C. Z., Burbidge A. A., Harrison P. L., Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proc. Natl. Acad. Sci. U.S.A. 112, 4531–4540 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho P., Studies in the Population of China, 1368-1953 (Harvard University Press, Cambridge, MA, 1959). [Google Scholar]
- 48.Wooldridge J. M., Introductory Econometrics: A Modern Approach (South-Western College Publishing, Mason, OH, ed. 5, 2012). [Google Scholar]
- 49.Teng S. N., Xu C., Sandel B., Svenning J.-C., Effects of intrinsic sources of spatial autocorrelation on spatial regression modelling. Methods Ecol. Evol. 9, 363–372 (2018). [Google Scholar]
- 50.Hao Z., et al. , Spatial patterns of precipitation anomalies in eastern China during centennial cold and warm periods of the past 2000 years. Int. J. Climatol. 36, 467–475 (2016). [Google Scholar]
- 51.He Y., [Historical Changes in Distributions of Tigers and Bears of China] [in Chinese] (Hunan Normal University Press, Changsha, China, 1996).
- 52.Koch P. L., Barnosky A. D., Late quaternary extinctions: State of the debate. Annu. Rev. Ecol. Evol. Syst. 37, 215–250 (2006). [Google Scholar]
- 53.Nogués-Bravo D., Ohlemüller R., Batra P., Araújo M. B., Climate predictors of late quaternary extinctions. Evolution 64, 2442–2449 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Smith F. A., Elliott Smith R. E., Lyons S. K., Payne J. L., Body size downgrading of mammals over the late Quaternary. Science 360, 310–313 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Ruddiman W. F., Earth’s Climate: Past and Future (Freeman, New York, ed. 3, 2013). [Google Scholar]
- 56.Intergovernmental Panel on Climate Change , Climate Change 2014: Synthesis Report Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Intergovernmental Panel on Climate Change, Geneva, 2014). [Google Scholar]
- 57.Brook B. W., Sodhi N. S., Bradshaw C. J. A., Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Cao Z., “Tigers and humans: A study on anthropogenic factors in distribution of tigers in China and its historical changes” PhD thesis, Shaanxi Normal University, Shaanxi, China (2010). (in Chinese).
- 59.Chapron G., et al. , Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1519 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Svenning J.-C., et al. , Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc. Natl. Acad. Sci. U.S.A. 113, 898–906 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Courchamp F., et al. , The paradoxical extinction of the most charismatic animals. PLoS Biol. 16, e2003997 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perino A., et al. , Rewilding complex ecosystems. Science 364, eaav5570 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Manfredo M. J., et al. , Why social values cannot be changed for the sake of conservation. Conserv. Biol. 31, 772–780 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Ceauşu S., Graves R. A., Killion A. K., Svenning J. C., Carter N. H., Governing trade-offs in ecosystem services and disservices to achieve human-wildlife coexistence. Conserv. Biol. 33, 543–553 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Bruskotter J. T., Wilson R. S., Determining where the wild things will be: Using psychological theory to find tolerance for large carnivores. Conserv. Lett. 7, 158–165 (2014). [Google Scholar]
- 66.Malhi Y., The concept of the Anthropocene. Annu. Rev. Environ. Resour. 42, 77–104 (2017). [Google Scholar]
- 67.Hobbs R. J., et al. , Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 15, 1–7 (2006). [Google Scholar]
- 68.Boyd R., Richerson P. J., The Origin and Evolution of Cultures (Oxford University Press, New York, 2005). [Google Scholar]
- 69.Mesoudi A., Whiten A., Laland K. N., Towards a unified science of cultural evolution. Behav. Brain Sci. 29, 329–347, discussion 347–383 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Wen R., The Distributions and Changes of Rare Wild Animals in China (Shandong Science and Technology Press, Jinan, China, 2009) (in Chinese). [Google Scholar]
- 71.Wan X., et al. , Historical records reveal the distinctive associations of human disturbance and extreme climate change with local extinction of mammals. Proc. Natl. Acad. Sci. U.S.A. 116, 19001–19008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkinson E., Chinese History: A New Manual (Harvard University Asia Center, Cambridge, MA, ed. 5, 2018). [Google Scholar]
- 73.Ge Q., Zheng J., Hao Z., Liu Y., Li M., Recent advances on reconstruction of climate and extreme events in China for the past 2000 years. J. Geogr. Sci. 26, 827–854 (2016). [Google Scholar]
- 74.Chamberlian G., Analysis of covariance with qualitative data. Rev. Econ. Stud. 47, 225–238 (1980). [Google Scholar]
- 75.Stammann A., bife: Binary Choice Models with Fixed Effects. R package, Version 0.4. https://cran.r-project.org/web/packages/bife/index.html (2017).
- 76.R Core Team , R: A language and environment for statistical computing (Version 3.4.1, R Foundation for Statistical Computing, Vienna, 2017). http://www.R-project.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.