Significance
The ability of HIV to rapidly generate escape mutations in response to host immune pressure constitutes a major obstacle toward the development of an efficacious vaccine. Assessing how easily the virus evades different selection pressures remains challenging because it requires quantifying how frequently escape mutations emerge across a diverse virus population. Barcoded SIV infection in nonhuman primates allowed for the direct assessment of escape dynamics within ∼4,000 distinct barcoded viral lineages that established infection. Over 1,000 viral lineages survived cytotoxic T-lymphocyte selection and were confirmed to harbor escape mutations. Characterizing the genetic bottleneck resulting from selection (i.e., the number of surviving barcode lineages) provides a way to assess and compare the efficacies of different selective forces in eradicating the virus.
Keywords: genetic bottleneck, barcoded virus, viral adaptation, population dynamics, evolutionary biology
Abstract
The rapidity of replication coupled with a high mutation rate enables HIV to evade selective pressures imposed by host immune responses. Investigating the ability of HIV to escape different selection forces has generally relied on population-level measures, such as the time to detectable escape mutations in plasma and the rate these mutations subsequently take over the virus population. Here we employed a barcoded synthetic swarm of simian immunodeficiency virus (SIV) in rhesus macaques to investigate the generation and selection of escape mutations within individual viral lineages at the Mamu-A*01-restricted Tat-SL8 epitope. We observed the persistence of more than 1,000 different barcode lineages following selection after acquiring escape mutations. Furthermore, the increased resolution into the virus population afforded by barcode analysis revealed changes in the population structure of the viral quasispecies as it adapted to immune pressure. The high frequency of emergence of escape mutations in parallel viral lineages at the Tat-SL8 epitope highlights the challenge posed by viral escape for the development of T cell-based vaccines. Importantly, the level of viral replication required for generating escape mutations in individual lineages can be directly estimated using the barcoded virus, thereby identifying the level of efficacy required for a successful vaccine to limit escape. Overall, assessing the survival of barcoded viral lineages during selection provides a direct and quantitative measure of the stringency of the underlying genetic bottleneck, making it possible to predict the ability of the virus to escape selective forces induced by host immune responses as well as during therapeutic interventions.
The ability to quantify and molecularly characterize HIV RNA in plasma has provided crucial insights into HIV biology, evolution, pathogenicity, and transmission. The rapid and dynamic nature of HIV infection was first uncovered by modeling the kinetics of plasma viral loads after the initiation of drug therapy (1–3). Subsequently, studies characterizing the genetic composition of plasma virus during different stages of infection revealed that while infection is generally established by a single transmitted founder (T/F) virus (4, 5), the virus population rapidly accumulates vast sequence diversity as it adapts to selection pressures (6, 7). The ability of HIV to generate mutations that allow the virus to evade host immune responses prevents the eradication of the infection in most patients and constitutes a major obstacle toward the development of an effective vaccine (8). Tracking the dynamics of escape mutations once they have become detectable in plasma has shed light on the strength of the underlying selective pressures that drive the evolution of the virus population (9–13). However, investigating the stochastic processes underlying the emergence of escape mutations in plasma virus has remained difficult, in part because it has not been possible to directly track the evolutionary dynamics of the distinct viral lineages that make up the HIV quasispecies.
Escape from cellular immune responses involves first the generation of viral mutations that prevent the recognition of viral peptides by cytotoxic CD8+ T lymphocytes (CTLs) and the subsequent selection of these mutations in the viral population. The generation of new mutants occurs during reverse transcription of the viral genome and is therefore affected by the size of the replicating viral population. The subsequent selection of these mutants is determined by the strength and immunodominance of the immune response, the replicative fitness cost of the mutation, and factors such as the overall replication rate of the viral population (11, 13–18). The timing of CTL escape appears to play a major role in the rate of selection of escape variants. Escape mutants are selected very rapidly in acute infection, whereas slow selection is observed later in infection (17, 19, 20).
The rapid selection of immune escape variants in acute infection has major implications for HIV vaccination. There are strong reasons to suggest that vaccine immunogens should target conserved or late-escaping epitopes, since these may be more susceptible to immune control. However, the reasons for late escape at particular epitopes are often unclear. Late escape may occur because of difficulties in generating the required escape mutations, reduced replicative fitness of escape mutants, or simply delays until the immune response is strong enough to select variants. The emergence of escape mutants may take longer if several mutations (or compensatory mutations to increase replicative fitness) are required (21). However, if a single point mutation is sufficient for escape, it has been argued, although not directly demonstrated, that the timing of escape is primarily affected by the timing of the immune response, as the mutation rate is high enough that any single point mutation should be present simultaneously in multiple virus variants (22).
The mechanisms constraining immune escape are important to understand if vaccines are to target epitopes with a particular escape profile. If the rate of generation of new mutations is a limiting factor, then vaccines that reduce the effective population size of the virus (e.g., by controlling peak viral load) will reduce immune escape. However, if escape occurs late because the immune response is weak, nondominant, or delayed in natural infection, then a stronger and more rapid vaccine-induced response may simply drive earlier immune escape from vaccine-associated responses (16). In either case, it is likely that the early stages of infection in a vaccinated individual would involve a race between immune control of viral replication and the generation and selection of immune escape mutations. A major question is therefore how much immune control of viral replication would be required in acute infection to prevent the generation of escape mutations. That is, How low would a vaccine need to keep virus levels to prevent immune escape?
Quantifying the rate at which escape mutations are generated in the replicating virus population is crucial for predicting whether and when escape from a selective pressure is likely to occur. However, distinguishing low-frequency escape mutations (before they have been amplified by selection) from errors generated during next-generation sequencing is challenging. Furthermore, once escape mutations are unambiguously detectable in plasma, it is difficult to phylogenetically infer the dynamics underlying their emergence and selection in individual viral lineages to quantify the rate at which they were generated in the virus population. To overcome these challenges and increase the resolution of viral dynamics analyses, we recently developed a barcoded virus system (simian immunodeficiency virus [SIV]mac239M) that allows for the genetic discrimination of approximately 10,000 viral variants designed to vary only within a 34-bp insert between the vpx and vpr genes (23). The number and relative proportion of each barcode in any given specimen can be quantified by next-generation sequencing, allowing individual barcoded viral lineages to be tracked and monitored during infection of nonhuman primates. Importantly, if a barcode lineage persists in plasma during strong selection pressure, it has likely acquired an escape mutation. Therefore, assessing the survival of individual barcode lineages during selection may provide a way to directly quantify the stringency of the underlying genetic bottleneck. Here we utilized SIVmac239M to elucidate the dynamics underlying the emergence and subsequent selection of escape from a specific CTL response. The well-characterized Mamu-A*01-restricted Tat-SL8 epitope, which previous studies have shown to arise in early acute infection and to undergo early escape via multiple amino acid substitutions (24–28), provides an optimal experimental model to assess the feasibility of our approach.
Tracking the frequencies of distinct barcode lineages during the first weeks of SIV infection revealed the persistence of more than 1,000 different barcode lineages in the virus population during CTL selection. The parallel emergence of Tat-SL8 escape mutations in numerous distinct viral lineages was confirmed by single-genome sequence analysis, underscoring the ease of escape at this epitope. Intriguingly, several minor viral lineages increased substantially in frequency during immune selection, highlighting the stochastic nature of immune escape. Overall, using the barcoded virus system to study viral escape provided a window into the underlying selection landscape imposed by the Tat-SL8 specific CTL response, allowing us to directly quantify both the stringency of the genetic bottleneck and the level of viral replication associated with immune escape.
Results
Selection of Escape at Tat-SL8 Epitope.
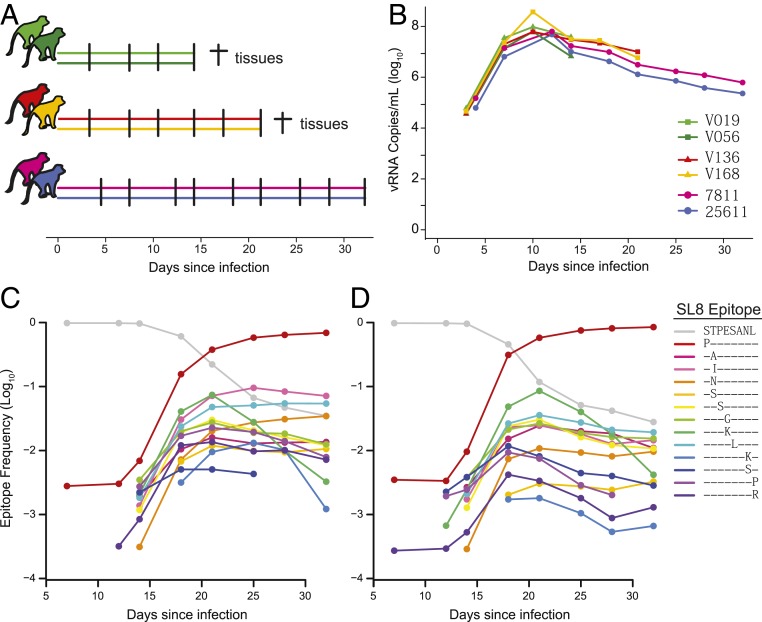
To investigate the dynamics of CTL escape at the Tat-SL8 epitope within individual viral lineages, 6 Mamu-A*01+ rhesus macaques were infected i.v. with 2.2 × 105 IU of SIVmac239M, with 2 animals necropsied each on day 14 postinfection (V019 and V056) or on day 21 postinfection (V136 and V168) and 2 animals followed for 32 d (25611 and 7811) (Fig. 1A). The plasma viral load trajectories were characteristic of SIVmac239 infection, peaking by day 12 postinfection and subsequently declining modestly in all animals (Fig. 1B). To assess the kinetics of escape at the Tat-SL8 epitope, we used next-generation sequencing to track the relative frequencies of the predominant escape mutations between day 7 and day 32 in animals 25611 and 7811 (Fig. 1 C and D). The frequencies of most escape mutants first exceeded the limit of detection of the assay at day 14, indicating that immune selection against the Tat-SL8 epitope was initiated within the second week of infection. The kinetics of escape proceeded rapidly, with escape mutants constituting the majority of plasma virus by day 21. The most frequent escape mutant in both animals involved a serine to proline substitution at the first position of the epitope (1P), in line with previous studies (24–28). All predominant escape mutations (reaching at least 1% relative frequency) were generated via single-nucleotide substitutions, consisting of a transition mutation (purine to purine or pyrimidine to pyrimidine).
Fig. 1.
Viral replication and immune escape in plasma. (A) Six animals were infected i.v. with 220,000 IU SIVmac239M on day 0 and followed for up to 32 d. Two animals were necropsied each on day 14 and day 21 with spleen, lymphoid, and GI tissues collected for sequence analysis. (B) Viral RNA copies were measured in the plasma of each animal. (C and D) The relative frequencies (log10) of wild-type (gray line) and all major Tat-SL8 escape mutants (reaching at least 1% frequency in either animal; colored lines) are shown for animals 25611 (C) and 7811 (D) at various sampled time points. Protein sequences of the escape mutations are shown in the keys. Frequencies below the determined limit of detection of each escape mutant are not shown.
Emergence of Tat-SL8 Escapes across Tissues.
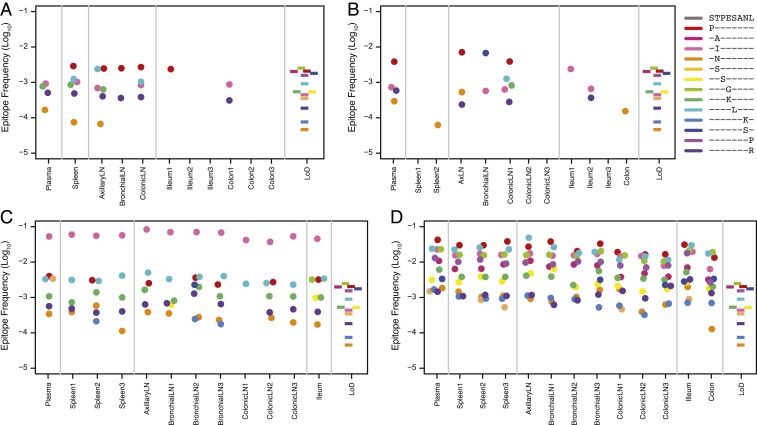
While plasma virus is thought to provide an overall representation of virus replicating in host tissues, differences in the distribution of target and CD8+ T cells may lead to heterogeneity in the generation and selection of CTL escape mutants across tissues. To assess the emergence and dissemination of Tat-SL8 escape mutants across the primary sites of viral replication in early infection, we compared the distribution of escape mutants across lymphoid and gastrointestinal (GI) tissues in animals necropsied on day 14 and day 21 (Fig. 2 A–D). Immune selection was evident in both animals necropsied on day 14, with several different escape mutants observed above the limit of detection in some lymphoid and GI tissues. Most escape mutants amplified in tissues were also detectable in plasma (4 of 6 in animal V019 and 4 of 7 in animal V056), confirming that plasma virus is representative of the different viral variants replicating in tissues in early infection. Numerous escape mutants were detectable in animals necropsied on day 21 (a total of 10 escape mutants in V136 and 13 escape mutants in V168) with the overall frequency of escape mutants in plasma nearly 10-fold higher than in animals necropsied on day 14. Furthermore, the predominant escape mutants in animals necropsied on day 21 exhibited similar frequencies across plasma and all sampled tissues, suggesting that while the generation of escape mutations is intrinsically stochastic, the anatomical distribution rapidly diminishes as viral lineages redistribute between tissues.
Fig. 2.
Immune escape in tissues. Shown are the relative frequencies (log10) of the predominant Tat-SL8 escape mutations (colored circles) (A–D) above the limit of detection in tissues for each necropsied animal. The protein sequences of the escape mutations are shown in the keys. The colored bars in the rightmost panels depict the range of sequencing error specific to each amino acid change among 6 replicates.
Escape Mutations Emerge Simultaneously in Distinct Viral Lineages.
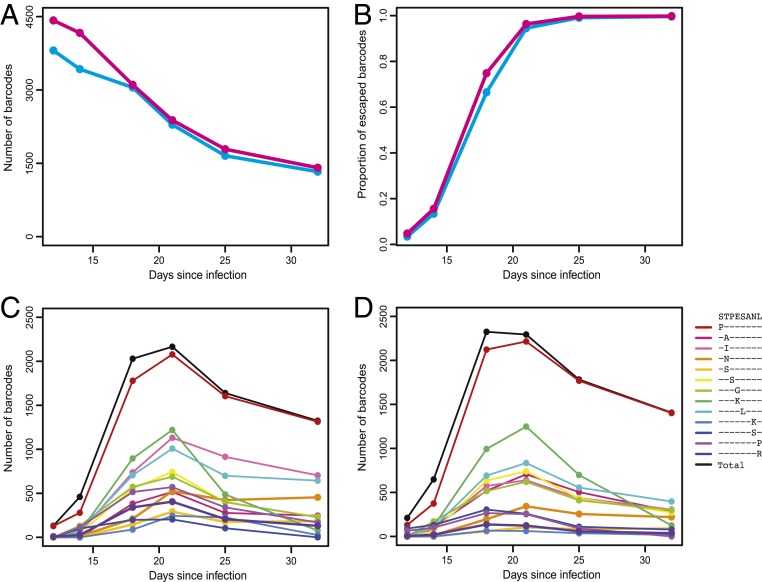
Tracking the dynamics of individual barcode lineages over time provides a means to quantify the ability of the virus population to escape selection pressure and characterize the underlying selection landscape at an unprecedented level of detail. To assess the stringency of the selection bottleneck imposed by the CTL response, we determined the number of distinct barcodes remaining detectable in plasma between days 12 and 32 for animals 25611 and 7811 (Fig. 3A). We observed a total of 4,519 and 5,078 distinct barcode lineages across all time points in animals 25611 and 7811, respectively, of which 3,807 and 4,422 were observed at peak viremia on day 12. Over 95% of the barcodes that were first observed after day 12 were at a low frequency (below 0.01%) and were therefore likely not detected at peak viremia due to input limitations. While the majority of barcodes were lost from observation during the overall postpeak decline in plasma viremia, concomitant with ongoing immune selection, 1,329 (35%) and 1,409 (32%) distinct barcode lineages remained detectable on day 32 in animals 25611 and 7811, respectively. The persistence of more than 1,000 detectable barcode lineages after selection suggests widespread emergence of escape mutations in parallel viral lineages.
Fig. 3.
The persistence of barcode lineages indicates immune escape. (A) The number of observed barcodes at each time of sampling is shown for animals 25611 (cyan) and 7811 (pink). (B) The fraction of barcode lineages harboring Tat-SL8 escape mutations (at frequencies exceeding the limit of detection for linkage of our assay) at each time point is shown for each animal. (C and D) The number of barcode lineages harboring any escape mutations (black lines) and specific escape mutations (colored lines, with protein sequences shown in the key) at each time point is shown for animal 25611 (C) and animal 7811 (D). Barcode lineages with escape mutations at frequencies below the limit of detection for linkage of our assay are excluded.
To confirm that the persistence of barcode lineages reflects underlying immune escape, we next assessed the linkage between the genetic barcode and the Tat-SL8 epitope, which is retained during deep sequencing due to the proximity of the respective genomic regions. We first determined the fraction of barcode lineages that had acquired any escape mutations at the Tat-SL8 epitope over time (Fig. 3B). In both animals, the fraction of barcode lineages harboring escape mutations increased proportionately with the overall frequency of escape mutants in the virus population, with all detectable barcode lineages having escaped by day 32. In these analyses, we accounted for potentially confounding PCR-induced recombination artifacts by quantifying the within-PCR error rate in our assay and determining the limit of detection for each escape mutation based on its frequency in plasma at each time point (Methods). We next employed single-genome amplification (SGA)-based sequencing, which is not subject to potential PCR-induced recombination artifacts (4), to corroborate the emergence of Tat-SL8 escape mutations in numerous parallel viral lineages. In line with estimates obtained using next-generation sequencing, SGA revealed that 109 of 119 (92%) distinct barcode lineages were linked to escape mutations on day 21 in animal 25611, and 124 of 201 (62%) barcode lineages were linked to escape mutations on day 18 in animal 7811.
To assess the distribution of different escape mutants among surviving barcode lineages, we determined the number of distinct barcode lineages that had acquired each predominant escape mutant over time (Fig. 3 C and D). During the dominant immune selection time frame (between day 12 and day 21), each escape mutant became detectable in an increasing number of barcode lineages as it amplified in frequency. Overall, the population-level frequency of each escape mutant at day 21 was highly correlated with the number of distinct barcode lineages in which it was detected (ρ = 0.98 in animal 25611 and ρ = 0.97 in animal 7811; Spearman’s rank correlation). The total number of barcode lineages harboring escape mutations declined after day 21, suggesting that the subsequent dynamics of viral lineages after the selective sweep at the Tat-SL8 epitope were driven by emergence of other selective pressures or genetic drift. These results support our premise that the persistence of barcode lineages during selection reflects escape and thereby provides a quantifiable measure of the underlying selective bottleneck.
Size of Barcode Lineage Determines Persistence during Selective Sweep.
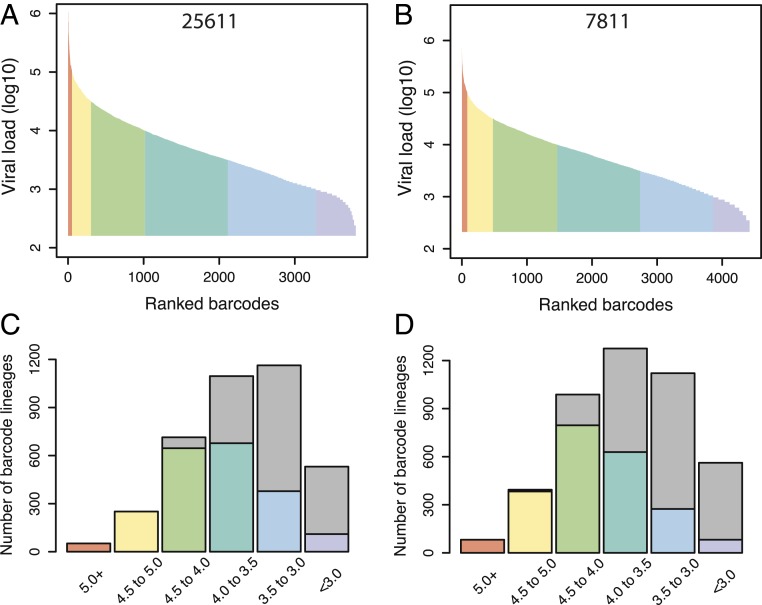
The emergence of escape mutations in the virus population depends on the rate at which the required mutation(s) is generated during reverse transcription and the size of the replicating viral population. Using a barcoded virus to study immune escape makes it possible to track the replication dynamics and persistence of thousands of individual barcode lineages during selection. This model provides a means to estimate the level of viral replication required for the emergence of escape mutants. To this end, we first determined the viral loads attributable to all observed barcode lineages at day 12, before immune selection was evident in the virus population (Fig. 4 A and B). The peak viral loads exhibited a dynamic range of over 3 orders of magnitude in both animals, with a median of 3.6 log10 RNA copies per milliliter per lineage in animal 25611 and 3.7 log10 RNA copies per milliliter per lineage in animal 7811. We next assessed the proportion of barcode lineages that survived the selective sweep occurring at the Tat-SL8 epitope and remained detectable in plasma on day 21, partitioned based on their peak viral loads at day 12 (Fig. 4 C and D). The persistence of barcode lineages increased with their size at day 12, with 90% of barcode lineages with peak viral loads of at least 4.0 log10 RNA copies per milliliter remaining detectable on day 21 while only 17% of barcode lineages with peak viral loads of less than 3.0 log10 RNA copies per milliliter persisted in the 2 animals.
Fig. 4.
The peak viral loads of barcode lineages predict persistence. (A and B) Individual barcode lineages were rank ordered based on the frequency in animals 25611 (A) and 7811 (B) at day 12 (x axis). Then the viral loads (log10) for each lineage are estimated at peak viral load (proportion times total viral load at peak) (y axis). The color coding demarcates 0.5 log10 intervals in viral load between 5.0 and 3.0 log10. (C and D) For barcodes grouped according to estimated viral load at peak viremia, we observed the number of barcodes persisting until day 21 for animals 25611 (C) and 7811 (D) (colored bars). Gray bars indicate the number of barcode lineages “lost” between peak and day 21 in each baseline viral load category.
To assess the level of viral replication required for immune escape, we next used a maximum-likelihood approach to estimate the per virion probability of persistence of individual barcode lineages from day 12 to day 21 in the 2 animals followed longitudinally (Methods). The estimated probability of persistence was 0.00018/1 RNA copy per milliliter (present at day 12). In other words, a viral load of 3.6 log10 RNA copies per milliliter is required for a barcode lineage to have a 50% chance of gaining at least one Tat-SL8 escape mutation and persisting to be detected at day 21. However, because the population bottleneck imposed by target cell limitation around peak viremia is expected to result in a loss of both wild-type virus and escape mutants, barcode lineages may require multiple copies of the Tat-SL8 escape mutant to survive both immune selection and drift. Overall, our findings highlight the ease with which escape mutations are generated at the Tat-SL8 epitope and the stochastic nature of viral replication in acute infection.
The Trajectories of Individual Barcode Lineages Reflect Underlying Population Dynamics of Escape.
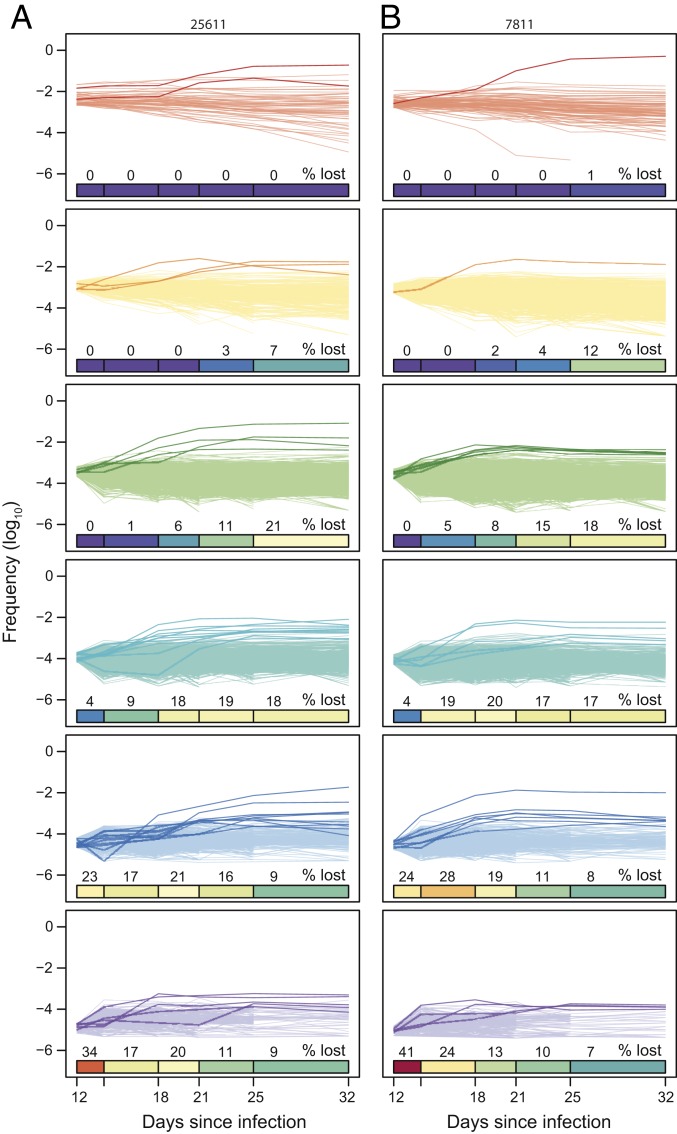
The persistence of over 1,000 distinct barcode lineages during CTL escape at the Tat-SL8 epitope revealed a soft selective sweep consisting of multiple reoccurring mutations (29), in contrast to a classic hard selective sweep, where a single beneficial mutation arises in the population and then increases to fixation (30). While low genetic diversity in acute SIV infection makes it difficult to detect subtle signatures of adaption in viral sequences associated with soft selective sweeps, tracking the dynamics of individual barcode lineages over time allows us to directly assess how the composition of the virus population changed as it adapted to immune selection. To this end, we plotted the relative frequencies of all observed barcodes over time, partitioned based on their peak viral loads (Fig. 5). The smaller the peak viral load of a barcode, the earlier in infection it declined below the limit of detection of our assay. The loss of higher-frequency barcodes from detection primarily occurred after the selective sweep at the Tat-SL8 epitope (day 21), again indicating the development of other selective forces later in acute infection. Most surviving barcode lineages exhibited modest adjustments in relative dominance between day 12 and day 21, with 87.9% of barcode lineages in animal 25611 and 85.5% of barcode lineages in animal 7811 either declining or increasing in frequency at most 2-fold. In a notable departure from this overall pattern, a small subset of barcode lineages, several of which were at a low frequency at peak viremia, increased more than 10-fold in relative dominance in each animal (highlighted lines in Fig. 5), suggesting that these lineages had an overall fitness advantage during selection compared to other surviving barcode lineages.
Fig. 5.
The longitudinal dynamics of barcode lineages during immune selection. The relative frequencies (log10) of barcode lineages are shown over time for animals 25611 (A) and 7811 (B) partitioned based on their peak viral loads at day 12. The color coding demarcates 0.5 log10 intervals in viral load between 5.0 and 3.0 log10. The heat bars show the cumulative percentage of barcodes that are lost to detection over time. Barcode lineages that increase at least 10-fold in relative frequency between day 12 and day 21 are highlighted by thicker and darker lines.
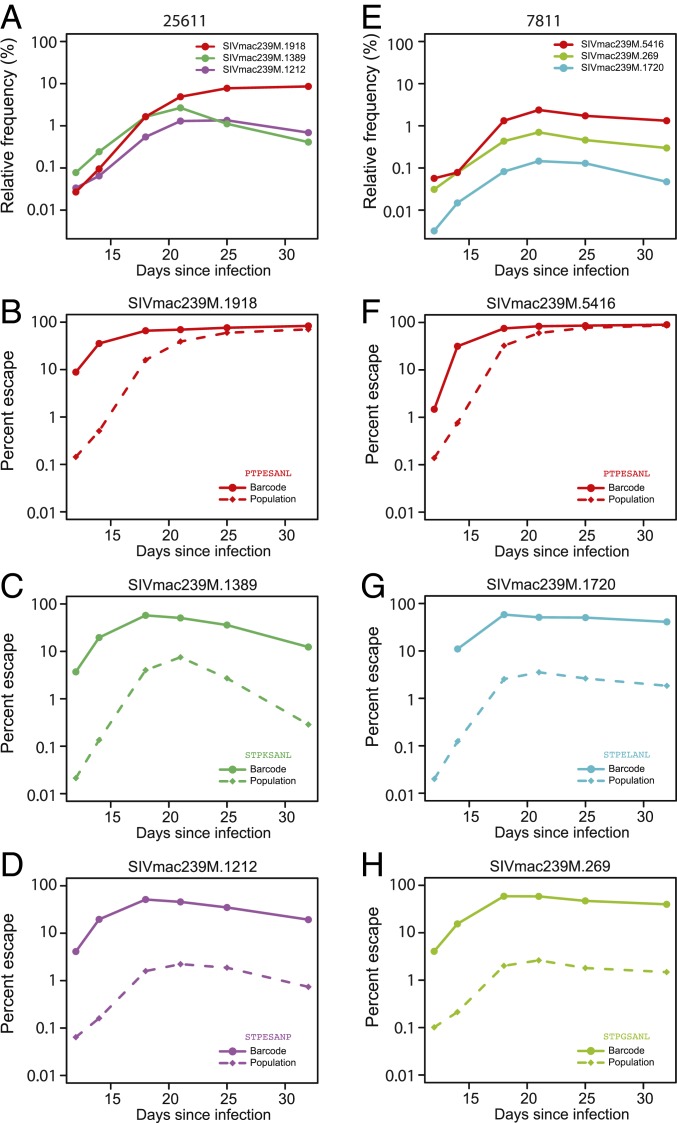
We next investigated whether the different trajectories of surviving barcode lineages could be explained by underlying differences in the emergence and dynamics of escape at the Tat-SL8 epitope and, therefore, differences in average fitness during selection. Specifically, the average relative fitness of each barcode lineage at the start of selection should depend on the fraction of escaped viruses in that lineage compared to the virus population on average. To this end, we assessed the fraction of viruses within individual barcode lineages harboring Tat-SL8 escape mutations at day 18, when the mutations first became detectable but not yet fixed in lineages. As expected, barcode lineages that increased in frequency during selection (between day 12 and day 21) had significantly higher fractions of escape mutations at day 18 than lineages that either declined in frequency or remained relatively static (66.9% vs. 35.4% with P value = 2.0E-7 in animal 25611; 79.7% vs. 51.9% with P value = 2.6E-12 in animal 7811; Wilcoxon rank-sum test). To further investigate how the dynamics of barcode lineages were shaped by the underlying dynamics of escape, we next tracked the frequency of the predominant escape mutation over time in several amplified barcode lineages in which escape mutations were detectable early in infection (Fig. 6). As the predominant escape mutation increased in frequency within a barcode lineage (Fig. 6 C and D), the trajectory of the barcode lineage increasingly resembled the trajectory of the escape mutant at the population level (Fig. 6 A and B). Interestingly, the proportion of escape mutations was 10- to 100-fold higher in the amplified barcode lineages than in the virus population overall in the early days of selection (day 12 and day 14), implying that they had acquired escape mutations earlier than other barcode lineages on average. Overall, tracking the dynamics of individual barcode lineages during CTL escape at the Tat-SL8 epitope captured changes to the structure of the virus population, reflecting the underlying stochasticity in the dynamics of escape within individual surviving viral lineages.
Fig. 6.
The dynamics of barcode lineages reflect the underlying dynamics of escape. Shown are the relative frequencies of select barcode lineages for animal 25611 (A) and animal 7811 (E). The color of the lines corresponds to the predominant escape mutation in each barcode, with each amino acid change indicated in B–D and F–H, respectively. The relative frequencies of the predominant escape mutation within each barcode lineage (solid lines) and in the virus population overall (dashed lines) are shown for animal 25611 (B–D) and animal 7811 (F–H).
Discussion
Our current understanding of the emergence and subsequent expansion of escape mutations under different selective forces in HIV/SIV infection has largely been derived from population-level measures, including the time to detectable escape after the onset of selection and the overall rate at which escape mutations overtake wild-type virus. Here we employed a synthetic swarm consisting of ∼10,000 barcode-discriminable but otherwise isogenic clones to increase the resolution of analysis of the dynamics of escape at the Mamu-A*01-restricted Tat-SL8 immunodominant epitope to the level of individual barcoded viral lineages. This study examined CTL escape within individual viral lineages, allowing us to assess the recurrent emergence of escape mutations and track changes to the population structure of the viral quasispecies as it adapted to selection pressure.
We observed more than 1,000 different barcode lineages surviving selection pressure in each of 2 longitudinally followed animals, implying widespread concurrent emergence of escape mutations for the Tat-SL8 epitope in distinct viral lineages. Barcode lineages surviving selection were directly confirmed to harbor escape mutations through next-generation and SGA-based sequencing. Overall, the emergence of escape mutations at the Tat-SL8 epitope in numerous parallel viral lineages is consistent with the rapid and diverse dynamics of escape documented previously (24–28) and is further corroborated by our observation of escape mutations across GI and lymphoid tissues within 2 to 3 wk of infection. As such, the proportion of barcode lineages that survive selection pressure provides a direct and relative measure of the stringency of the underlying bottleneck.
The high frequency of emergence of escape mutations in parallel viral lineages highlights the challenge posed by viral escape for the development T cell-based vaccines. Conventional T cell-based vaccines, for which vaccine-induced T cell responses decay from postvaccination peak levels to lower levels of memory T cells, require time for proliferation, differentiation, and trafficking following antigen reexposure before reaching full effectiveness. This delay allows a period of early viral replication, with viral loads reaching ∼105 copies per milliliter prior to the onset of effective responses (31). In contrast, vaccines that employ cytomegalovirus (CMV) as a persistent vaccine vector elicit indefinitely persistent, broadly tissue-distributed, effector memory-differentiated T cell responses, ensuring the presence of virus-specific T cells prior to the expansion of the virus population in acute infection (32, 33). Interestingly, sustained low-level viral replication has been observed in CMV-vaccinated animals which later went on to fully suppress virus (32, 33), whereas nonprotected animals showed typical unperturbed viral dynamics. This suggests that if viral loads can be kept below a certain threshold, immune responses can control and ultimately eradicate the infection. However, beyond that threshold, SIV mutation allows the virus to escape selection forces. Precisely estimating the level of viral replication required for the generation of escape mutations is therefore a crucial component of effective vaccine design.
Using a barcoded virus to track the survival of a large number of viral lineages of different sizes facilitates estimating the probability of escape in viral populations of different sizes. For example, Fig. 4 shows that for the Tat-SL8 epitope, 100% of barcode lineages with >105 copies per milliliter at peak viremia escaped, and even for relatively minor lineages between 103 and 103.5 copies per milliliter, ∼28% escaped. Because of the large number of barcode lineages competing, a delay in the emergence of escape in a viral lineage would have meant that the lineage was “left behind” by the selection of other variants, limiting its subsequent contribution to the viral quasispecies. A delay of even a few days would have rendered the smallest barcode lineages undetectable by our sequencing, implying that the probability of escape at low viral loads may be even higher than estimated. Overall, further work is required to more precisely identify the viral population size conducive to immune escape mutation and the mechanisms of immunity required to maintain viral loads below this level.
The small viral loads required for Tat-SL8 escape are not unexpected in light of the high probability of generating at least one point mutation somewhere in the viral genome during each reverse transcription event and the fact that a number of different single point mutations can confer escape at the epitope. In addition to de novo mutation, a barcode lineage can acquire an escape mutation through recombination. While the template switching rate of HIV is high [∼10 events per reverse transcription (34, 35)], only ∼2% of recombination events are expected to affect the linkage between the barcode and the Tat-SL8 epitope due to their close proximity (223 nucleotides apart). Additionally, because recombination requires coinfection with 2 strains, the probability of any 2 strains recombining is proportional to their frequency in the population. Therefore, while the escape mutation remains at a low frequency, so does the probability of coinfection and recombination. In other words, a barcode lineage harboring an escape mutation would need to reach a high level in the virus population before substantial recombination with other barcode lineages would be possible. Consequently, acquiring an escape mutation through recombination likely involves a considerable delay. Since there is no clear mechanism by which barcode lineages with delayed escape would subsequently catch up with lineages that had acquired escape mutations earlier during selection, we postulate that de novo mutation, rather than recombination, constitutes the major mechanism by which Tat-SL8 escape mutations arose in parallel viral lineages. Resolving the relative contributions of de novo mutation and recombination to viral adaptation remains an important area of future work. Tracking the emergence of different escape mutations in individual barcode lineages may shed light on these processes.
Despite the relatively nonrestrictive selection bottleneck imposed by CTL pressure targeting the Tat-SL8 epitope, based on relatively low genetic barriers and fitness costs for escape, tracking the dynamics of individual barcoded lineages over time revealed important changes to the structure of the virus population during selection. While most surviving barcode lineages exhibited limited changes in relative dominance, a small number of barcode lineages expanded considerably in frequency. These increases in the proportions of distinct lineages are not due to intrinsic qualities of the barcode itself but appear to be directly attributable to the proportion of Tat-SL8 escape mutations during selection in those lineages, reflecting heterogeneity in the timing of immune escape between individual lineages. Furthermore, genetic drift during the period of viral load decline in acute infection and potential secondary fitness effects such as reversion of suboptimal mutations on the SIVmac239 backbone (36) or the onset of other immune responses targeting epitopes elsewhere on the viral genome may further contribute to the observed heterogeneity in the dynamics of individual barcode lineages.
We envision that by providing unprecedented resolution into the structure of the virus population, the barcoded virus system can be used to characterize how the complex selective landscape induced by simultaneous and sequential cellular and humoral immune responses shapes viral adaption in natural infection. We expect that the more challenging it is for the virus population to escape a particular selection pressure, the fewer barcode lineages will remain detectable in plasma, providing a relative measure of how close the virus population came to eradication. Assessing the dynamics of barcode lineages during chronic infection from immune responses targeting epitopes that may be challenging to escape or require compensatory mutations to retain replicative fitness will be important. Overall, using a barcoded virus to quantify the proportion of individual viral lineages persisting during selective pressure provides a high-resolution, standardized measure of the underlying genetic bottleneck, which may be used to assess, compare, and predict the outcome of different host immune responses or therapeutic interventions acting on the viral population.
Methods
Ethics Statement.
Animals were handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All Institutional Animal Care and Use Committee (IACUC) guidelines were followed and all procedures were performed according to protocols approved by the IACUC of the National Cancer Institute (assurance A4149-01) under the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Six Indian-origin male rhesus macaques (Macaca mulatta) were housed at the National Institutes of Health (NIH) in Animal Biosafety Level 2 housing with routine husbandry practices as described previously (23). All animals tested negative for SIV, simian type-D retrovirus, cercopithecine herpesvirus 1, and simian T-lymphotropic virus type 1 at the beginning of the study.
Animal Study.
Six animals were infected i.v. with 2.2 × 105 IU (1 mL) of transfection-produced SIVmac239M titered on TZM-bl cells, as previously described (23). Two animals were necropsied at day 14 and 2 animals were necropsied at day 21 to assess the distribution of barcodes and Tat-SL8 escape mutations across GI, lymphoid, and spleen tissues. Two animals were followed longitudinally for 32 d to simultaneously track the dynamics of individual barcode lineages and CTL escape at the Tat-SL8 epitope.
In Vivo Sample Collection.
As previously described (23), whole blood was collected in EDTA Vacutainer tubes (BD) from sedated animals, after which plasma for viral RNA quantification was separated from whole blood by centrifugation and stored at 80 °C.
Plasma Viral Load Determination.
Quantitative real-time PCR was used to quantify SIV RNA plasma viral loads (with a limit of quantification of 15 viral-RNA copies per milliliter) at all sampled time points as described previously (37).
Tissue Nucleic Acid Extraction and Quantification.
Various lymphoid and gut tissues were collected at necropsy and dissected into 1-cm3 pieces and snap frozen. Tissue pieces were homogenized in 1 mL of TriReagent (Molecular Research Center) in 2-mL extraction tubes of Lysing Matrix D (MP Biomedicals) using Precellys 24 (Bertin Instruments). A phenol extraction protocol was used to extract viral RNA as previously described (38). Recovered RNA was dissolved in minimal volumes of 10 mM Tris⋅Cl, pH 8.0, for qRT-PCR as well as for use in various sequencing approaches described below.
MiSeq Sample Preparation.
RNA samples were prepared for MiSeq sequencing as previously described (23). RNA isolation from plasma or viral stock was performed using a QIAamp Viral RNA mini kit according to manufacturer’s instructions, with isolated RNA eluted using a 65-μL elution buffer. Superscript III reverse transcriptase (Invitrogen) and a gene-specific reverse primer SL8R (5′-AGC TGA GAG AGG ATT TCC TCC C-3′) at position 6,408 to 6,429 were used to synthesize cDNA from the extracted DNA (23). The reaction mixture was prepared as previously described (23) with initial incubation at 50 °C for 1 h, followed by incubation at 55 °C for an additional hour, and incubation at 70 °C for 15 min. Each reaction was then treated with RNaseH and incubated at 37 °C for 20 min. Quantification of the cDNA synthesized in the previous step was performed via qRT-PCR using the primers VpxF1 (5′-CTA GGG GAA GGA CAT GGG GCA GG-3′) at 6,079 to 6,101 and SL8R (23). The cDNA was then amplified using bulk PCR, with MiSeq adaptors added directly onto the amplicon. High-Fidelity Platinum Taq or Platinum Taq (Thermo Fisher Scientific) was used to prepare the reactions according to the manufacturer’s instructions. As previously described (4), the reactions were performed with the VpxF1 and SL8R primers combined with either the F5 or F7 Illumina adaptors containing unique 8-nucleotide index sequences, under the following reaction conditions: 94 °C, 2 min; 40 × (94 °C, 15 s; 60 °C, 1.5 min; 68 °C, 30 s); 68 °C, 5 min. The number of input templates ranged from 5 × 103 copies to 1 × 106 copies. The multiplexed samples and PhiX library were then prepared via standard protocols as previously described (23).
Single-Genome Amplification.
Single-genome amplification followed by direct Sanger sequencing was used to assess linkage between the Tat-SL8 epitope and the barcode on RNA isolated from plasma on day 21 in animal 25611 and day 18 in animal 7811. Following the extraction of viral RNA using the methodology described in the previous section, cDNA was synthesized using superscript III and the gene-specific primer SIVnFL.R1 (5′-CCC AAA GCA GAA AGG GTC CTA ACG-3′). Platinum Taq DNA Polymerase High Fidelity (Thermo Fisher Scientific) was used to perform nested PCR amplification. Briefly, 1× High-Fidelity Platinum PCR buffer, 2 mM MgSO4, 0.2 mM of each deoxynucleoside triphosphate, 0.2 μM of each primer, and 0.025 units/μL Taq were combined in a 20-μL reaction. The first-round PCR was then performed with SIVnFL.F1 (5′-GAT TGG CGC CYG AAC AGG GAC TTG-3′) and SIVnFL.R1 primers using a limiting dilution of cDNA prior to PCR amplification. The PCR was a denature step at 94 °C for 1 min followed by 35 cycles of 94 °C for 20 s, 55 °C for 30 s, and 68 °C for 1 min/kb and terminated with a single 10-min 68 °C extension. Next, 1 μL of each reaction was transferred to a second-round reaction which was amplified under the same PCR conditions for 45 cycles with the VpxF1 and SL8R primers. PCR reactions were assessed by gel electrophoresis. Positive wells were directly sequenced on an ABI 3730xl genetic analyzer using BigDye Terminator chemistry (Applied Biosystems).
Sequencing Analysis.
Sequences were demultiplexed based on exact matches to the Illumina P5 index (1 of 40). All sequences for each unique index read were then aligned to the first 28 bases of the vpr gene, allowing for 2 nucleotide mismatches. The 34 bases directly upstream of the start codon for vpr were extracted, corresponding to the barcode. The 24 bases corresponding to the Tat-SL8 epitope were also extracted from each sequence. The linkage between the barcode and the Tat-SL8 epitope was retained for each sequence because they were on a single amplicon. All reads with a quality score lower than 30, or with a barcode that did not exactly match one of the previously identified barcodes in the SIVmac239M stock (23), were excluded from future analyses. Since the number of template cDNA copies was quantified using qRT-PCR, the limit of detection for animal samples was set at the theoretical number of sequences resulting from a single template copy (one per template input). All barcode–epitope pairs with read counts below the limit of detection were removed, and the relative frequency of each pair was then determined for each sample.
Quantifying Limit of Detection for Different Tat-SL8 Epitope Variants.
We directly estimated the rate of generating each predominant amino acid escape mutation at the Tat-SL8 epitope during bulk PCR by sequencing the SIVmac239M stock in 6 replicates. We determined the mean and variance in frequency for each escape mutation and, assuming that error is log-normally distributed, used the 99th quantile as the limit of detection of the assay for each escape mutant.
Quantifying Limit of Detection for Linkage between Tat-SL8 Epitope and Barcode.
In vitro recombination during bulk PCR can artificially link an escape mutation at the Tat-SL8 epitope barcode to a barcode on a different genomic background. We estimated the level of recombination directly by mixing 50:50 two specifically generated clones with a different barcodes and different escape mutants (PTPESANL and SIPESANL). For barcode 1, there were 1,463,449 reads of SIPESANL with 104,054 reads of PTPESANL. For barcode 2, there were 1,462,267 reads of PTPESANL with 96,148 reads of SIPESANL. Since silent recombination between 2 copies of the same sequence should account for 50% of total in vitro recombination, the rate of mismatched linkage gives an overall in vitro recombination rate of 13%. Consequently, we cannot rule out artificial linkage of a barcode lineage to an escape mutation through in vitro recombination during bulk PCR if the fraction of the escape mutation in the barcode lineage does not exceed 13% of the overall frequency of the mutation in the plasma virus population. In all analyses, we considered a Tat-SL8 escape mutation to have been acquired by a barcode lineage only if the fraction of the mutation within the lineage exceeded the specific error threshold of the assay for the escape mutation at the time of sampling.
Statistical Analysis.
All statistical analyses were performed in R version 3.3.1. To assess whether the frequency of each predominant escape mutant in plasma was significantly correlated with the number of distinct barcode lineages in which it was detected, we performed Spearman’s rank correlation test (using the base cor.test function). We used a maximum-likelihood approach to estimate the per virion probability of retention from day 12 to day 21. We assumed that the number of persisting virions per barcode lineage was binomially distributed, with probability of retention per virion p, and number of trials n corresponding to the closest integer value of the total peak viral load attributable to each barcode. We then used Brent’s method implemented in the base optim function (with a lower bound of 0 and an upper bound of 1) to minimize the negative of the log likelihood, where the first term corresponds to barcode lineages that remained detectable in plasma on day 21 and the second term to barcode lineages that were no longer detectable in plasma on day 21. Finally, we assessed whether the fraction of Tat-SL8 escape mutations was significantly higher at day 18 in barcode lineages that increased at least 10-fold in frequency between day 12 and day 21 compared to all other persisting barcode lineages using the Wilcoxon rank-sum test.
Data Availability.
All data are included in the main text and within SI Appendix.
Supplementary Material
Acknowledgments
We thank the nonhuman primate care staff in the Laboratory Animal Sciences Program, Frederick National Laboratory for Cancer Research for expert animal care. We also thank the Nonhuman Primate Research Support Core, the Quantitative Molecular Diagnostics Core, and the Viral Evolution Core of the AIDS and Cancer Virus Program, Frederick National Laboratory for Cancer Research for specimen processing, viral load analysis, and sequencing. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contracts HHSN261200800001E and 75N91019D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. R.W.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914967117/-/DCSupplemental.
References
- 1.Perelson A. S., Ribeiro R. M., Modeling the within-host dynamics of HIV infection. BMC Biol. 11, 96 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho D. D., et al. , Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373, 123–126 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Wei X., et al. , Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373, 117–122 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Keele B. F., et al. , Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U.S.A. 105, 7552–7557 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giorgi E. E., et al. , Estimating time since infection in early homogeneous HIV-1 samples using a Poisson model. BMC Bioinformatics 11, 532 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffin J. M., HIV population dynamics in vivo: Implications for genetic variation, pathogenesis, and therapy. Science 267, 483–489 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Shankarappa R., et al. , Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73, 10489–10502 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goulder P. J. R., Watkins D. I., HIV and SIV CTL escape: Implications for vaccine design. Nat. Rev. Immunol. 4, 630–640 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Fernandez C. S., et al. , Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J. Virol. 79, 5721–5731 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asquith B., Edwards C. T., Lipsitch M., McLean A. R., Inefficient cytotoxic T lymphocyte-mediated killing of HIV-1-infected cells in vivo. PLoS Biol. 4, e90 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Althaus C. L., De Boer R. J., Dynamics of immune escape during HIV/SIV infection. PLoS Comput. Biol. 4, e1000103 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganusov V. V., et al. , Fitness costs and diversity of the cytotoxic T lymphocyte (CTL) response determine the rate of CTL escape during acute and chronic phases of HIV infection. J. Virol. 85, 10518–10528 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganusov V. V., De Boer R. J., Estimating costs and benefits of CTL escape mutations in SIV/HIV infection. PLoS Comput. Biol. 2, e24 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balamurali M., et al. , Does cytolysis by CD8+ T cells drive immune escape in HIV infection? J. Immunol. 185, 5093–5101 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Petravic J., Loh L., Kent S. J., Davenport M. P., CD4+ target cell availability determines the dynamics of immune escape and reversion in vivo. J. Virol. 82, 4091–4101 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davenport M. P., Loh L., Petravic J., Kent S. J., Rates of HIV immune escape and reversion: Implications for vaccination. Trends Microbiol. 16, 561–566 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Kijak G. H., et al. , Rare HIV-1 transmitted/founder lineages identified by deep viral sequencing contribute to rapid shifts in dominant quasispecies during acute and early infection. PLoS Pathog. 13, e1006510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh L., Batten C. J., Petravic J., Davenport M. P., Kent S. J., In vivo fitness costs of different Gag CD8 T-cell escape mutant simian-human immunodeficiency viruses for macaques. J. Virol. 81, 5418–5422 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh L., Petravic J., Batten C. J., Davenport M. P., Kent S. J., Vaccination and timing influence SIV immune escape viral dynamics in vivo. PLoS Pathog. 4, e12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goonetilleke N., et al. ; CHAVI Clinical Core B , The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 206, 1253–1272 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelleher A. D., et al. , Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J. Exp. Med. 193, 375–386 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martyushev A. P., et al. , Epitope-specific CD8+ T cell kinetics rather than viral variability determine the timing of immune escape in simian immunodeficiency virus infection. J. Immunol. 194, 4112–4121 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Fennessey C. M., et al. , Genetically-barcoded SIV facilitates enumeration of rebound variants and estimation of reactivation rates in nonhuman primates following interruption of suppressive antiretroviral therapy. PLoS Pathog. 13, e1006359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen T. M., et al. , Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407, 386–390 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Vanderford T. H., et al. , Viral CTL escape mutants are generated in lymph nodes and subsequently become fixed in plasma and rectal mucosa during acute SIV infection of macaques. PLoS Pathog. 7, e1002048 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bimber B. N., et al. , Ultradeep pyrosequencing detects complex patterns of CD8+ T-lymphocyte escape in simian immunodeficiency virus-infected macaques. J. Virol. 83, 8247–8253 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E. Y., et al. , Contribution of CD8+ T cells to containment of viral replication and emergence of mutations in Mamu-A*01-restricted epitopes in Simian immunodeficiency virus-infected rhesus monkeys. J. Virol. 82, 5631–5635 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor D. H., et al. , Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8, 493–499 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Pennings P. S., Hermisson J., Soft sweeps II–Molecular population genetics of adaptation from recurrent mutation or migration. Mol. Biol. Evol. 23, 1076–1084 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Smith J. M., Haigh J., The hitch-hiking effect of a favourable gene. Genet. Res. 89, 391–403 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Davenport M. P., Ribeiro R. M., Perelson A. S., Kinetics of virus-specific CD8+ T cells and the control of human immunodeficiency virus infection. J. Virol. 78, 10096–10103 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen S. G., et al. , A live-attenuated RhCMV/SIV vaccine shows long-term efficacy against heterologous SIV challenge. Sci. Transl. Med. 11, eaaw2607 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen S. G., et al. , Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlub T. E., et al. , Fifteen to twenty percent of HIV substitution mutations are associated with recombination. J. Virol. 88, 3837–3849 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cromer D., Grimm A. J., Schlub T. E., Mak J., Davenport M. P., Estimating the in-vivo HIV template switching and recombination rate. AIDS 30, 185–192 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Alexander L., Denekamp L., Czajak S., Desrosiers R. C., Suboptimal nucleotides in the infectious, pathogenic simian immunodeficiency virus clone SIVmac239. J. Virol. 75, 4019–4022 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., et al. , Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 113, E3413–E3422 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deleage C., et al. , Defining early SIV replication and dissemination dynamics following vaginal transmission. Sci. Adv. 5, eaav7116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the main text and within SI Appendix.