Abstract
Introduction
Spinal cord injury (SCI) predisposes those who suffer from it to physical inactivity and weight gain; consequently, death due to cardiovascular diseases is more frequent among people with SCI than in the general population. The literature documents a consensus about an interdisciplinary multimodal approach for the prevention and treatment of cardiovascular risk factors including overweight and obesity in people with SCI, focusing on diet, physical activity (PA) and behavioural interventions. This study will investigate implementation of recommendations from a recent clinical practice guideline for identification and management of cardiometabolic risk after SCI through multimodal patient education in a subacute clinical setting.
Methods and analysis
All patients who are aged 18 years or older with an SCI within the previous 12 months and admitted to highly specialised rehabilitation are included, regardless of SCI aetiology or neurological level. A primary study designed as a controlled, pragmatic, preintervention- postintervention study with 6-month follow-up evaluates the effect of the clinical intervention; a prospective national cohort study on body mass index (BMI) serves as a historical control. The intervention consists of a standardised approach to patient education about cardiovascular risk factors, PA and a healthy diet that begins at the outset of primary SCI rehabilitation and is integrated into existing settings and workflows. Outcome measures are collected at admission, discharge and 6 months after discharge and include peak oxygen uptake (VO2peak) (primary outcome), BMI, body composition, metabolic profile, neurological status, level of functioning, depression, quality of life, objective PA (accelerometry), self-reported PA, self-assessed PA ability, shared decision making, and dietary habits. Test–retest reliability of four VO2peak test protocols are investigated, as is test–retest reliability of a multisensor accelerometer in a rehabilitation setting.
Ethics and dissemination
The project is approved by the Committees on Health Research Ethics in the Capital Region of Denmark on 10 July 2018 (Journal-nr.: H-18018325). The principal investigator obtains informed consent from all participants. The interventions in the project are closely related to existing rehabilitation care, and the risk of pain and discomfort is considered modest. Any unintended events related to the elements of the intervention are reported, according to existing regional procedures. Data are stored in a secure web-based database (Redcap). The primary study and prospective cohort study are registered at Clinicaltrials.gov. Positive and negative results will be submitted to relevant scientific journals related to SCI for publication. Important protocol modifications are reported to the Committees on Health Research Ethics in the Capital Region of Denmark.
Trial registration numbers
NCT03689023 and NCT03369080.
Keywords: spinal cord injuries, metabolic syndrome, health promotion, exercise, diet therapy
Strengths and limitations of this study.
The prospective cohort study includes both spinal cord injury (SCI) centres in Denmark and the intervention study includes all newly injured people with SCI admitted to rehabilitation at the East Denmark SCI-centre.
Four predefined protocols for assessing peak oxygen uptake are used due to the heterogeneity of functional level in the SCI population.
The preintervention and postintervention study is based on a pragmatic real-life approach by including existing settings and work flows, which is a strength, but consistent implementation of multimodal interventions may be challenging due to changes in the clinical setting.
Lack of randomisation is a study limitation.
Introduction
The annual incidence of traumatic spinal cord injury (SCI) in Denmark is 10–15 per million,1 while non-traumatic SCI has accounted for approximately 60% of all newly injured patients admitted to the two SCI centres in Denmark in recent years. SCI is a life-changing event that may affect all bodily functions below the level of the lesion, requiring highly specialised interdisciplinary rehabilitation aiming at the highest possible level of independent functioning and resulting in significant costs to affected individuals and society. Rehabilitation at the Clinic for Spinal Cord Injuries in Eastern Denmark (CSCI) generally includes functional training, strength training, cardiovascular exercise and fine motor training of the upper extremities. In addition, circulation, respiration, thermoregulation, bowel and bladder function, skin integrity, pain and spasticity are continually assessed and addressed, and aids are provided to compensate for functional losses, including communication aids and splinting. Counselling to address social and economic issues, sexual function and psychological issues is provided.
Over the long term, SCI and resulting impairments predispose affected individuals to increased cardiovascular risk and premature cardiovascular death; a clinical practice guideline addressing cardiometabolic disease after SCI was recently published.2 However, targeted patient education addressing long-term cardiovascular risk, based on individualised face-to-face interaction between patients and healthcare professionals and aiming at a core clinical outcome, is not systematically integrated into early stages of specialised SCI rehabilitation at CSCI, even though an opportunity may exist to target the link between injury-related immediate impacts on functionality and long-term health consequences.3 4 Similarly, health promotion education and activities related to body mass index (BMI), diet, smoking, alcohol intake and physical activity (PA) are not systematically provided, and assessment of physical capacity, metabolic profile and body composition is not a part of standard care. A systematic approach may ensure that all patients at CSCI receive information and knowledge related to health promotion and the risk of cardiovascular disease, which may support patient adaptation and adherence to recommended PA and healthy diet.
As a result, cardiovascular risk factors, including weight gain and the consequences of an inactive lifestyle during and after primary rehabilitation, are the focus of the current study.
Course of overweight
The prevalence of overweight in people with SCI is conservatively estimated at 66%. Overweight has been found to be one of the most common cardiometabolic risk factors among people with SCI, increasing the cardiovascular risk profile of wheelchair-dependent people with paraplegia.5–8 Energy expenditure decreases significantly after sustaining a SCI and remains low. Although body fat and body weight decrease in the acute injury phase, they increase in the subacute phase, and a loss of lean body mass in the lower extremities and trunk has been observed during the first year after injury.9 BMI increases gradually during the first years after discharge from primary rehabilitation.10 Obese people with SCI achieve a lower level of functioning during primary rehabilitation than do those of normal weight.11 Overweight in people with SCI is associated with increased risk of depression.12 Nutritional education delivered by a dietician or lifestyle coach has been found promising,13–15 although it is often not offered in a clinical setting.16 Increased knowledge about weight management among clinicians is recommended, but weight management is often not prioritised in rehabilitation settings. Clinicians have called for evidence-based knowledge and clinical guidelines.16 17
Impact of PA on health and fitness
In the general population, PA is associated with beneficial effects on diseases contributing to the metabolic syndrome, and its beneficial effect increases when it is combined with diet therapy.18 Similar effects of PA among people with SCI have been described; numerous studies have reported the positive effects of PA intervention programme in people with both acute and chronic SCI on physical capacity, strength and functional performance, including the effect of exercise interventions on cardiometabolic health.19 20 Evidence-based exercise guidelines for cardiometabolic health in people with SCI recommend a minimum of 30 min of moderate to vigorous aerobic exercise three times weekly to reduce cardiovascular risk factors.21 The long-term effect increases when PA is combined with behavioural interventions.22 However, not all people with SCI are able to participate in PA intervention programme or maintain PA. Rates of participation in leisure time PA and in sports activities after discharge from primary rehabilitation are low among people with SCI.23 24 Intrapersonal and extrapersonal factors influence participation in PA, including self-efficacy related to being physically active.25 PA alone is insufficient to induce weight loss in people with SCI.2 Therefore, a broader approach to cardiovascular risk reduction may be appropriate, and a combination of several interventions is required to promote a physically active lifestyle and weight loss.26 Examples of key intervention components are autonomy in relation to decision-making and behavioural interventions comprising goal setting and feedback via physical assessments.26 27
Although the separate or combined effects of PA, diet and behavioural interventions have been investigated previously in people with SCI with generally positive results, this study will investigate the effect of educational and behavioural interventions related to PA and diet in a subacute clinical rehabilitation setting. The study will investigate implementation of recommendations from the recent clinical practice guideline for identification and management of cardiometabolic risk after SCI, including assessments of physical capacity, body composition, bodyweight, dyslipidaemia and impaired fasting glucose, as well as PA and diet.2 Feedback on these assessments and goal setting will be part of the patient education delivered by clinical staff across settings during primary rehabilitation.
To the best of our knowledge, only a single study has investigated outcomes related to cardiovascular risk factors following PA and behavioural interventions during subacute inpatient rehabilitation using outcomes related to cardiovascular risk factors, but this study only included wheelchair users.28 29 The current study will contribute to existing knowledge by consecutively enrolling all patients, aged 18 years or older, with a new SCI who are admitted to CSCI, regardless of mobility status, and by evaluating the implementation of evidence-based guidelines for identification and management of cardiometabolic risk after SCI in a clinical setting.
Objectives
This study will investigate the effect of a systematic approach to incorporating targeted patient education about cardiovascular risk factors, PA and a healthy diet early in the primary rehabilitation process, compared with a historical control group.
Study design
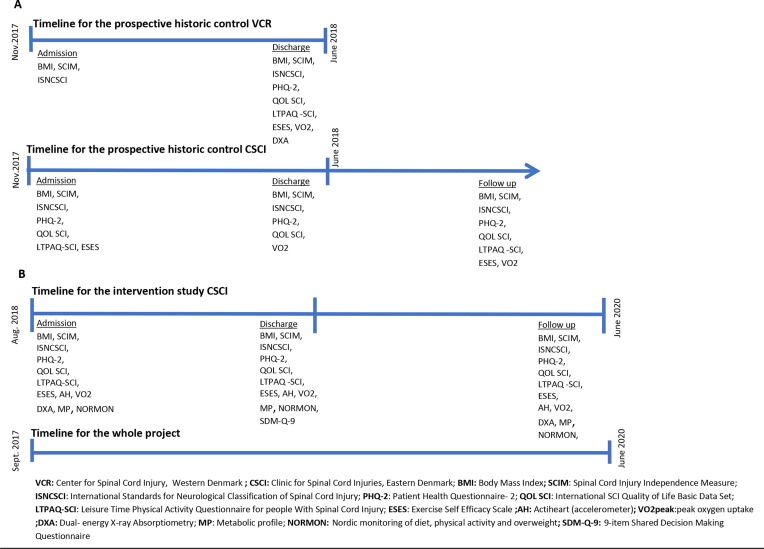
The primary study comprises a primary study designed as a controlled, pragmatic, preintervention-postintervention study with 6 months of follow-up. A prospective national cohort study provides a historical control (figure 1).
Figure 1.
Timeline for all substudies and used outcome measures. (A) and (B) illustrate the prospective historic control study and the intervention study, respectively.
Substudies
BMI is considered a high-risk determinant due to the impact of overweight on the cardiovascular risk profile and level of functioning among wheelchair-dependent people with SCI.5–8 A prospective representative longitudinal survey of BMI conducted before the controlled intervention in collaboration with SCI Center of Western Denmark serves as a historical control (substudy 1). Additional outcome measures will be collected at CSCI during the survey period, including measures of PA, physical capacity and body composition. Two substudies of test–retest reliability of a VO2peak test (substudy 2) and a multisensor accelerometer (substudy 3) will be performed. VO2peak and accelerometry are considered valid methods to measure the effect, amount and intensity of PA at discharge from primary SCI rehabilitation. Both will be collected repeatedly during the primary study and serve as individual motivational components in education and communication, as well as outcome measures. Assessing test–retest reliability of the two procedures is essential.
Methods and analysis
The Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines are used in the reporting of the clinical trial.30
Patient involvement
A user panel consisting of six patients (three women and three men aged 23–78 years), including both recently injured people and those who had been living with SCI for some time, was established and involved in the early phase of study protocol development. All participants were hospitalised at CSCI when they participated in semistructured focus group interviews about their perceptions of health promotion practices in the clinical setting.31 The interview focused on both the existing level of information about increased risk of overweight and cardiovascular disease after SCI and education about diet and PA as a way of reducing those risks. Data were analysed using constant comparative analysis.32 The user panel recommended more information in the early phase of rehabilitation about cardiovascular risk, PA and diet and more support and guidance about appropriate diet and being physically active, which is the primary aim of the project. The study results will be disseminated to project participants.
Participants and eligibility criteria
Inpatients who are aged 18 years or older, injured with SCI within the last 12 months and admitted at CSCI are recruited and consecutively included after providing informed consent, regardless of SCI aetiology (ie, traumatic or non-traumatic), neurological level or completeness (The completeness of the injury is graded according to the ASIA Impairment Scale (AIS)). AIS is used to determine the degree of motor and sensory function below the level of the SCI. A complete or incomplete injury is defined as absence or presence of sensory and motor function in the most caudal sacral segments. A=sensory and motor complete; B=sensory incomplete without motor function >3 levels below the motor level of injury on both sides of the body; C=motor incomplete, with preserved motor function below the level of injury and where >50% of the key muscles below the injury level have a degree <3 by manual muscle test (MMT); D=motor incomplete with preserved motor function below the level of injury and where >50% of key muscles below the injury level have a degree >3 by MMT; E=normal sensory and motor function in all segments. of the lesion). In substudy 1, all newly injured people with SCI admitted at the SCI Centre of Western Denmark are also included. Substudy 1 serves as a historical control group, and the intervention in the primary study is part of a new standard of care. Therefore, randomisation, blinding and sample size calculation are not appropriate.
Exclusion criteria for the VO2peak test include motor complete SCI (AIS A and B) at cervical (C) four level or above and a need for artificial ventilation. Other exclusion criteria are the presence of decubiti, severe spasticity or musculoskeletal problems at risk of exacerbation or aggravation during testing or preventing completion of the test.
Substudy 3 includes a convenience sample of 20 patients with the goal of ensuring variation in age, gender, neurological level and completeness of SCI.
Primary study: a systematic interdisciplinary multimodal intervention that facilitates PA, healthy diet and maintenance after discharge through strategic patient education as part of usual care, with the aim of decreasing cardiovascular risk.
This prestudy and poststudy includes all patients aged 18 years or older with a new SCI who are admitted at CSCI during a period of 12–18 months. The study includes follow-up 6 months after discharge from primary rehabilitation.
Approximately 70 patients with a new SCI are admitted to CSCI annually; but due to expected missing data, complete data sets from admission through follow-up may be fewer.
Intervention
The intervention is based on recommendations in a recently released clinical practice guideline for the identification and management of cardiometabolic risk after SCI and conclusions from a meta- synthesis by Williams et al and a systematic review by Greaves et al.2 26 27 A combination of several interventions is most effective at promoting a physically active lifestyle and weight loss after SCI. Crucial intervention components are autonomy in relation to decision-making about PA, support and follow-up from healthcare professionals and mentors with SCI, information about adapted PA and behavioural interventions comprising goal setting and feedback from, for example, physical tests. Greaves et al recommend group sessions, individual sessions and interdisciplinary interventions in the clinical setting that focus on maintaining PA and healthy diet.26
The intervention will be integrated into usual care during the project period, and all newly injured patients will receive all multimodal components as appropriate to individual circumstances, such as level of injury. At discharge, the patient will describe adherence to the intervention and document participation in targeted education elements on a checklist. Similarly, healthcare professionals will use a checklist to document adherence to interventions at the start, midpoint and end of the study period. Medical records and schedules for goal-setting meetings will also be reviewed to monitor healthcare professionals’ adherence to the interventions. Rehabilitation of the physical level of functioning and physical capacity (eg, physiotherapy) will occur as part of usual care and is a mandatory core component of highly specialised SCI rehabilitation. However, decisions about PA made by the patient during rehabilitation may be integrated into the rehabilitation programme to achieve his or her goals for PA during and after the rehabilitation period.
A central part of the intervention is to create a standardised approach to targeted strategic patient education of patients about cardiovascular risk factors, PA and a healthy diet by systematising the existing clinical setting and treatment interventions.
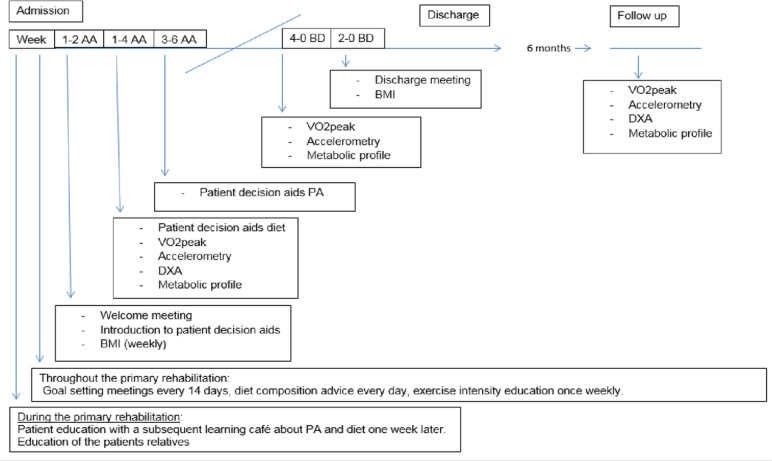
In the process of reorganising the institutional approach to addressing cardiovascular risks, pre-education of interdisciplinary healthcare personnel and peers with SCI is mandatory to clarify their roles in relation to targeted patient education. Pocket cards with evidence-based recommendations related to PA, diet and BMI in people with SCI are provided to all healthcare professionals and peers with SCI and will also illustrate the timeline for systematic targeted approaches during primary rehabilitation (figure 2).
Figure 2.
Timeline illustrated on the pocket card for systematic targeted approaches during primary rehabilitation within 2, 4 and 6 weeks after admission (AA) and during the last 4 and 2 weeks before discharge (BD) and at follow-up 6 months after discharge. BMI, body mass index; DXA, dual-energy X-ray absorptiometry; PA, physical activity; VO2peak, peak oxygen uptake.
Patients receive information and instructions about PA and healthy diet through patient education based on principles that include individualised face-to-face interaction between patients and healthcare professionals while working towards a specific health-related outcome.33 The interventions begin at the outset of primary SCI rehabilitation and are integrated into usual care at predetermined time points (eg, dual-energy X-ray absorptiometry (DXA) scan, VO2peak, metabolic profile with feedback early after admission to rehabilitation and goal setting meetings about PA and diet within 6 weeks after admission) throughout the entire rehabilitation continuum, with the goal of secondary and tertiary cardiovascular prevention.
Representatives of all the healthcare professions generally carry out education of patients and their relatives in a variety of educational settings,34 35 with a focus on clarifying the importance of PA and a healthy diet. Patient education involves training sessions36 and feedback on physiological outcome measures and tests that also serve as motivational tools. Additionally, goal-setting meetings, tools for shared decision making26 37 38 and use of mentors with SCI are also integrated as components supporting decision making about PA and healthy diet. BMI and diet are evaluated 3 months after discharge in an outpatient setting (see online supplementary appendix for a more detailed description of the strategic interventions).
bmjopen-2019-030310supp001.pdf (50.4KB, pdf)
All components are offered to patients as a mandatory part of the intervention, ensuring that information and patient education are provided and decisions about PA and healthy diet are made. However, patients individually determine the extent to which they engage in making decisions and setting goals about PA and healthy diet. Interdisciplinary healthcare professionals respect the decisions and autonomy of patients who choose not to set goals or make decisions about PA and healthy diet.
Several outcome measures used to evaluate the intervention at admission, discharge and at follow-up 6 months after discharge are also motivational components of the intervention: BMI, body composition measured by DXA, physical capacity (VO2peak), PA (Actiheart multisensor accelerometer) and blood samples describing metabolic profile.
Substudy 1: prospective national survey of BMI among people with SCI
This study includes all patients with a new SCI hospitalised at CSCI or SCI Centre of Western Denmark during a period of 10 months; 100 patients are expected to participate. Data on BMI, level of functioning (Spinal Cord Injury Independence Measure III (SCIM III)) and neurological status (International Standards for Neurological Classification of SCI (ISNCSCI) are collected at both centres. Patients with an SCI within the last 12 months who are admitted for rehabilitation several months after injury are also included in the prospective survey. Data on BMI at the time of injury are collected for all patients at admission to primary rehabilitation from the patient’s medical record. At CSCI, BMI every 6 weeks, quality of life (QoL SCI), depression (Patient Health Questionnaire-2 (PHQ-2)), amount of PA (Leisure Time Physical Activity Questionnaire for people with Spinal Cord Injury (LTPAQ-SCI)) and self-assessed ability to be physically active (Exercise Self Efficacy Scale (ESES)) will be collected at admission, discharge and follow-up 6 months after discharge. Measures of physical capacity (VO2peak) and body composition (DXA) are also obtained at discharge. Data from this substudy serve as a historical control for the intervention study.
Substudy 2: test–retest reliability of VO2peak testing
This study includes all patients participating in substudy 1 who are able to perform the VO2peak test at discharge from primary rehabilitation. Patients are randomised to a test session of either intrarater or inter-rater reliability. Due to the complexity of SCI, four predefined exercise protocols are used to reach criteria for VO2peak, defined as a respiratory exchange ratio >1.0.39 As a starting point, people with an incomplete SCI, as defined by ISNCSCI, will use a seated cross-trainer (NuStep T5XR), which has software incorporating both a standard and a modified test protocol. The standard protocol starts at 50 W with 25 W incremental increases every 2 min in the first three stages, 30 W increments thereafter and 115 steps per minute (SPM). The modified protocol starts at 25 W with 15 W increments every 2 min and 80 SPM. The equipment and modified protocol are reliable in people with traumatic brain injury and has been validated in healthy persons.40 41 In people with an incomplete SCI, the equipment is safe and involves a large amount of muscle mass.42 People with an ISNCSCI-defined complete SCI, very de-conditioned patients or those with an incomplete SCI but a poor ISNCSCI lower extremity motor score that may hinder reaching VO2peak on the seated cross trainer will use an arm-cranking ergometer (SCI FIT Pro1). Test protocols used on the SCI FIT ergometer are established from the most common protocols for people with tetraplegia and paraplegia during rehabilitation reported in a recent systematic review.39 The study protocols are designed as stage protocols starting at 5 W with an increase every minute of 5 W for people with tetraplegia and 10 W for people with paraplegia and 60 revolutions per minute.
If predefined criteria for VO2peak are not reached during test 1, a more suitable protocol to reach VO2peak is chosen for test 2 and will be retested at test 3. However, this is not possible if the protocol designed for people with tetraplegia is used. The test–retest study takes place at discharge, with 48 hours to 5 days between tests occurring at the same time of the day. Participants refrain from caffeine, alcohol and intensive physical exercise on the day of testing, as well as tobacco smoking 2 hours before testing. Bladder emptying occurs immediately before testing.
In the intervention study, the four exercise protocols are used to ensure that a true VO2peak is reached during the rehabilitation process. VO2peak is highly dependent on the level and completeness of the SCI and the testing equipment; for instance, a patient may be initially tested on the protocol designed for people with a complete tetraplegia and later tested on the non-modified standard protocol in the seated cross trainer due to neurological recovery and improvement in functional level.
Substudy 3: test–retest reliability of a multisensor accelerometer
This study includes a convenience sample of 20 patients ensuring a representative sample of individuals with paraplegia and tetraplegia, complete and incomplete SCI, age and gender. The equipment used for monitoring the amount and intensity of PA consists of sensors registering acceleration and heart rate and is placed on the thorax of the participant with two surface electrodes. The sensor can be dismantled from the thorax without removing the adhesive part of the surface electrodes, making it possible to easily reattach the sensor and resume monitoring after, for example, sleeping or bathing. Data are expressed as total and daily PA energy expenditure (kcal/min) and the time spent in different activity intensities on the basis of metabolic equivalents. The equipment has been previously used among wheelchair-dependent people with SCI, although its reliability in an inpatient setting has not been assessed.43 Precision is higher when the equipment is calibrated to individual participants using measures of energy expenditure and corresponding heart rate during rest and during exercise testing, covering a range of submaximal and maximal intensities. The equipment software uses these data to estimate energy expenditure using branched model equations.44 This method will also take into account compromised cardiac sympathetic innervation in individuals with an injury above T6. In this study, individual calibration is based on activity performed during the VO2peak test (substudy 2), with resting metabolic rate measured before testing for 10 min following a rest period of 20 min.45 46 In order to reliably measure total energy expenditure (kcal/min) and the amount and intensity of PA patients are instructed to wear the equipment for 48 hours. They are informed to take off the sensor (not the adhesive part) when bathing, but if they experience discomfort or skin irritation related to the equipment they can as well remove the adhesive part of the electrode. If they have impaired or absent sensation, they are recommended to take off the equipment when sleeping, and to check for skin irritation regularly, alternatively asking a nurse for help if they are not able to do this themselves. A period of 48 hours with sampling epochs every 15 s and a minimum wear-time of 80% is aimed for, and considered an appropriate wear time as described by Nightingale et al.47 However, data from recordings with <80% wear time will be analysed as well. To ensure comparability, test–retest procedures are performed over a period of 2 weeks on identical days of the week.
Outcome measures
Outcome measures evaluating the intervention comprise the following.
Primary outcome
Oxygen uptake is measured as VO2peak during a maximal exercise test and is the gold standard for measuring aerobic capacity. For people with SCI, several test protocols have been used.36
Secondary outcomes
Objective PA is measured in a subsample of the historical control cohort and participants in the intervention study with a multisensor device (Actiheart) recording accelerations and heart rate. It has been previously used for wheelchair users with SCI, and individual calibration is important to get the most accurate data.47 Evidence-based exercise guidelines for cardiometabolic health in people with SCI recommend a minimum of 30 min of moderate to vigorous aerobic exercise three times weekly.21
Bodyweight is measured as BMI, which is the most widely used outcome measure for body weight in people with SCI. BMI is not sensitive enough to distinguish between fat mass and lean body mass or overweight in people with SCI. Overweight among adults with SCI is defined as ≥22 kg/m2.2 48 BMI is already collected as part of usual care, and data for BMI every 6 weeks until discharge will be included in the project.
Body composition is determined by DXA, which is the gold standard for assessing obesity and body composition. Among adults with SCI, men with >22% body fat and women with >35% body fat should be classified as obese.2
Metabolic profile consists of C reactive protein (CRP) as a marker for inflammation and lipid profile including total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), which are included in the international SCI Endocrine and Metabolic Function Basic Data Set. Triglycerides should not be ≥150 mg/dL (1.7 mmol/L). HDL-C should not be <40 mg/dL (1.03 mmol/L) in men or <50 mg/dL (1.29 mmol/L) in women.2 LDL-C should not be >3.0 mmol/L.49 Haemoglobin A1c (HbA1c) serves as a marker for carbohydrate metabolism and is included in the International SCI Endocrine and Metabolic Extended Data Set.50 51 Criteria for a diagnosis of pre-diabetes include HbA1c 5.7%–6.4% (39–47 mmol/mol) and criteria for a diagnosis of diabetes include HbA1c>6.5% (>48 mmol/mol).2 As approved by the Committees on Health Research Ethics in the Capital Region of Denmark, blood samples will not be stored after analysis.
Blood pressure (BP) is measured by sphygmomanometry. Criteria for a diagnosis of hypertension in people with SCI vary with injury level, severity and aetiology. BP should not exceed 130/85 mm Hg.
Level of functioning is determined by the SCIM III, which is a valid and reliable outcome measure designed to assess level of functioning in people with SCI in clinical care and research.52–55
Neurological status is determined by the ISNCSCI and is the most widely used classification in people with SCI.56 57
Depression is measured by the PHQ-2, which is a generic measure of depression. Among people with SCI, a cut-off score of 3 is associated with sensitivity of 83.3% and specificity of 95.7%.58
QoL is measured by the International SCI QoL Basic Data Set (QoL SCI), which consists of three questions about satisfaction with life in general and physical and mental health. It is a valid outcome measure with good internal consistency.59 60
Self-reported PA is measured by the LTPAQ-SCI, which is a self-administered questionnaire about leisure time PA, including amount and intensity during the past 7 days. Reliability and validity of self-reported activity are satisfactory in moderate and high intensity levels.61 An additional question concerning PA outside of leisure time PA (ie, PA as part of rehabilitation) is included in substudy 1. The question is designed to be similar to the original questions and is scored using the same intensity scale. During the intervention study, a version of LTPAQ-SCI adjusted to a Danish context will be used. This version is approved by the developers of the original LTPAQ-SCI and includes active transportation and active physiotherapy.
Self-assessed ability to be physically active is measured by the ESES for people with SCI. It is an outcome measure developed for assessing self-efficacy related to PA in people with SCI and consists of 10 questions on a 0–4 response scale. ESES is reliable with high internal consistency (Cronbach’s alpha 0.94) and satisfactory content validity in the form of face and construct validity.62
Shared decision making related to patient decision aids for PA and healthy diet is measured by the 9-item Shared Decision Making Questionnaire (SDM-Q-9), which assesses the process of shared decision making between healthcare professionals and the patient from the patient’s perspective. SDM-Q-9 consists of nine statements, which can be rated on a six-point scale from 0 to 5, with higher scores indicating greater shared decision making. All items are summed to yield a raw total score of 0–45. SDM Q-9 is only used at discharge.
Varied and healthy diet in an appropriate amount is measured by the Nordic monitoring of diet, PA and overweight (NORMON) developed in a Nordic collaboration and commonly used for monitoring.63 The questionnaire explores how frequently 16 food indicators, several of which are recommended in the Nordic national nutritional recommendations, have been consumed over the previous 12 months. NORMON also includes questions related to alcohol intake, smoking and PA. The questionnaire was validated in 2009 against existing questionnaires about diet.64 In this study, a modified version of the questionnaire will ask patients to recall their dietary habits over the previous month.
Statistics
All data collected at admission, discharge and follow-up are continuous and are reported descriptively. In the intervention study, differences in the primary and secondary outcomes between baseline and follow-up will be analysed using analysis of covariance. The same approach will be used between baseline and follow-up in the historic control study. Likewise, differences between the intervention study and the historic control is analysed using analysis of covariance. Due to the small sample size, participants in the intervention study and historic control will not be matched but participants will be compared with each other controlling for ISNCSCI classification, gender and functional level. Linear regression is used to measure the strength and association between BMI and DXA results and the association between the psychometric variables, for example, QoL and depression compared with VO2peak and BMI. Ordinal regression analysis is made for ordinal data, for example, ESES. Missing data are analysed as intention to treat without imputation, but dropout analysis is made for primary outcomes. In substudies 3 and 4, the reliability of the outcome measures are analysed by paired t-test, Pearson’s product-moment correlation and coefficient of variation or intraclass correlation coefficient between the test–retest sessions.
Ethics and dissemination
During the intervention period, all newly injured patients who are admitted for rehabilitation at CSCI are offered treatment and tests included in the intervention as a mandatory part of usual care to the extent they are able to participate, which may vary with the level of lesion and completeness of SCI. Because the intervention is a part of usual care and comprises a standardised approach to patient education, no data monitoring or interim analysis is planned. Informed consent is obtained to analyse the data generated during the project. The intervention in the project is closely related with the content of the present rehabilitation, and the risk of pain and discomfort is considered modest. During the VO2peak test, special attention is paid to potential symptoms of autonomic dysreflexia (AD) in people with SCI above T5-6. In case of AD, the exercise test is stopped and relevant actions are initiated. It is assumed that any risks are surpassed by therapeutic gains, that is, expected reductions in the risk of cardiovascular disease and mortality. Any unintended events related to the intervention are reported according to existing regional procedures, and compensation is covered by the normal procedures for unintended harm during hospitalisation. The study is reported to the Danish Data Protection Agency and is registered at Clinicaltrials.gov (see WHO Trial Registration Data Set V.1.3.1) (table 1). Positive and negative results will be submitted to relevant scientific journals related to SCI for publication.
Table 1.
WHO trial registration data set (V.1.3.1)
| Data category | Information32 |
| Primary registry and trial identifying number | ClinicalTrials.gov (NCT03369080) and (NCT03689023) |
| Date of registration in primary registry | 12 November 2017 and 26 September 2018 |
| Secondary identifying numbers | The Committees on Health Research Ethics in the Capital Region of Denmark on 10 July 2018 (Journal-nr.: H-18018325) and the Danish Data Protection Agency (j.nr. VD-2018–380, I-Suite nr.: 6631) and (RH-2017–345, I-Suite nr.: 06052) |
| Source(s) of monetary or material support | This work was supported by a mutual cooperation about the research programme ‘Centre for Integrated Rehabilitation of Cancer Patients (CIRE)—Neuro/Psychology,’ between the University Hospitals Centre for Health Care Research, University hospital Copenhagen, Rigshospitalet, University College Copenhagen, Department of Nursing and Nutrition, and the NeuroScience Centre, Rigshospitalet |
| Primary sponsor | This work was supported by a mutual cooperation about the research programme ‘Centre for Integrated Rehabilitation of Cancer Patients (CIRE)—Neuro/Psychology,’ between the University Hospitals Centre for Health Care Research, University hospital Copenhagen, Rigshospitalet, University College Copenhagen, Department of Nursing and Nutrition, and the NeuroScience Centre, Rigshospitalet |
| Secondary sponsor(s) | |
| Contact for public queries | Nicolaj Jersild Holm (nicolaj.jersild.holm@regionh.dk), phone: +45 38631963, Rigshospitalet, Neuroscience Center, Clinic for Spinal Cord Injuries, Havnevej 25, 3100, Hornbæk, Denmark |
| Contact for scientific queries | Nicolaj Jersild Holm (nicolaj.jersild.holm@regionh.dk), phone: +45 38631963, Rigshospitalet, Neuroscience Center, Clinic for Spinal Cord Injuries, Havnevej 25, 3100, Hornbæk, Denmark |
| Public title | Health promotion and cardiovascular risk reduction in people with spinal cord injury—physical activity, healthy diet and maintenance after discharge: study protocol for a national cohort study followed by a clinical intervention study |
| Scientific title | Health promotion and cardiovascular risk reduction in people with spinal cord injury—physical activity, healthy diet and maintenance after discharge: study protocol for a prospective national cohort study followed by a preintervention and postintervention study |
| Countries of recruitment | Denmark |
| Health condition(s) or problem(s) studied | Spinal cord Injury and the cardiovascular risk factor, including weight gain and consequences of an inactive lifestyle during and after the primary rehabilitation |
| Intervention(s) | A controlled premultimodal and postmultimodal pragmatic clinical intervention study, with 6 months of follow-up containing ‘new usual care’ consisting of a uniform and systematic institutional strategy incorporating targeted strategic patient education about cardiovascular risk factors, physical activity and a healthy diet lifestyle starting early in the primary rehabilitation process |
| Comparator: a historic control conducted as a national prospective cohort study before ‘new usual care’ | |
| Key inclusion and exclusion criteria | Inclusion criteria: all patients who are 18 years or older, and with a spinal cord injury (SCI) within the last 12 months and admitted at clinic for spinal cord SCIs, are included regardless of aetiology to the SCI, neurological level or completeness of the lesion if informed consent is retrieved |
| Exclusion criteria: exclusion criteria for VO2peak test in the study includes motor complete SCI at C4 level or above, and assisted ventilatory function. Other exclusion criteria are the presence of decubitus, severe spasticity or musculoskeletal problems considered at risk of aggravation during testing or preventing completion of the VO2peak test | |
| Study type | Interventional: the study consists of a primary study designed as a controlled preclinical and postclinical intervention study and a historic control conducted as a prospective cohort study |
| Allocation: the intervention in the primary study is a part of new standard care. Therefore randomisation or blinding is not appropriate | |
| Primary purpose: prevention | |
| Date of first enrolment | November 2017 |
| Target sample size | 160 |
| Recruitment status | Recruiting |
| Primary outcome(s) | Oxygen uptake measured as VO2peak |
| Key secondary outcomes | Body mass index, body composition (determined by dual energy X-ray absorptiometry), metabolic profile consisting of C reactive protein as a marker for inflammation, lipid profile describing total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol and haemoglobin A1c as a marker for carbohydrate metabolism, and blood pressure |
| Ethics Review | The project is approved by the Committees on Health Research Ethics in the Capital Region of Denmark on 10.07.2018 (Journal-nr.: H-18018325) |
| Completion data | June 2020 |
| IPD sharing statement | Data can be accessed by request to the corresponding author after publications related to the PhD project are made |
Data statement section
All patient data are stored in a secure web-based database (Redcap) with limited access and ID code, to which data are transferred directly or by an encrypted USB stick. Patients are assigned unique identification numbers, which is the only identifier exported from Redcap during data analysis. Data are stored until 31 December 2027, after which paper material is shredded, data files are deleted and the Redcap database is no longer accessible. The principal investigator has access to all trial data. Data can be accessed on request to the corresponding author after reports related to the PhD project are published. No data monitoring committee is established.
Discussion
This study will investigate the effectiveness of a systematic institutional strategy incorporating individualised patient education and testing about cardiovascular risk factors, PA and a healthy diet lifestyle early after SCI diagnosis during primary rehabilitation, compared with a historical control group. Our findings will be discussed in light of recent studies suggesting that an interdisciplinary multimodal approach in prevention of cardiovascular risks among people with SCI with a focus on diet, PA and behavioural interventions is beneficial.13 14 23 25 65 66 Crucial components of the intervention are autonomy in relation to decision-making and support and follow-up from healthcare professionals and mentors with SCI. A qualitative meta-synthesis concluded that timely information about PA and its benefits in relation to SCI and behavioural interventions using goal setting and motivational feedback through physical tests might be important patient-activating tools.27 This is consistent with a recent systematic review by Greaves et al,26 who also strongly recommended that interventions in the clinical setting contain both group sessions and individual sessions as well as interdisciplinary interventions that focus on maintaining PA and healthy diet.26 These elements are incorporated into the intervention investigated in this study.
Several of the outcome measures used to evaluate the intervention are components of the intervention, as recommended in the clinical guideline for identification and management of cardiometabolic risk after SCI.2 Outcome measures also serve as individual motivational tools. The primary outcome measure is VO2peak, for which a significant positive relationship exists with some cardiometabolic markers in people with SCI, such as lipid profiles and fasting insulin levels.61 Consequently, PA that increases physical capacity may also reduce the risk of cardiovascular disease.67 Physical capacity measured as VO2peak is positively associated with functional independence,68 less physical strain during activities of daily living69 and life satisfaction70 among people with SCI, although other measures of physical capacity have an important and, in some cases, stronger impact on functional independence.68
Among people with SCI, several test protocols have been used for assessing VO2peak.36 In this study, four exercise protocols make VO2peak testing feasible for clinical physiotherapists who, although trained in using the testing equipment, are inexperienced in determining the appropriate workload during VO2peak testing, which is difficult due to the complexity of a SCI. If predefined criteria for VO2peak are not reached, a more suitable protocol is selected. The protocol and equipment used in the study are identical at admission and discharge. If a patient’s neurological and functional level has improved to the point where a different protocol and equipment will more accurately measure VO2peak, an additional test at discharge will be performed on a separate day. Data from both tests will be evaluated and the new protocol will be repeated at follow-up 6 months after discharge. This approach to testing VO2peak in a clinical setting has, to the best of our knowledge, not been described previously.
Secondary outcome measures include PA. Objective PA will be measured by the Actiheart accelerometer, which has previously been used for wheelchair users with SCI in laboratory and outpatient settings.47 In this study, it will be used in an inpatient setting and among people with SCI and some ambulatory function, which has not been previously described. As a measure of self-reported PA, a validated Danish version of the LTPAQ-SCI will be used. This version has been adapted to a Danish context in close collaboration with the developers of the original questionnaire; PA-related active transportation, such as hand biking or wheeling to work or school, as well as active physiotherapy exercises are included, as both are common PA for people with SCI in Denmark.
The primary study is possible due to the average length of stay during initial rehabilitation at CSCI, which is 85 and 86 days, respectively, for people with incomplete tetraplegia and paraplegia and 110 and 123 days, respectively, for people with complete tetraplegia and paraplegia (Fin Biering-Sørensen: Data from Clinic for Spinal Cord Injuries, Denmark, 2014). The study is highly dependent on adherence by interdisciplinary healthcare professionals and patients to the new intervention. Healthcare professionals’ adherence to the intervention is both supported and measured by a process inspired by a prospective effect and process evaluation for complex trials, in which at least 75% must agree that a specific element of strategic patient education has become a part of routine clinical practice before it is considered implemented.71 This evaluation is repeated every 6–8 weeks throughout the intervention period. Similarly, perceived barriers to implementation are also evaluated every 6–8 weeks throughout the intervention period. Interdisciplinary coordination meetings occurring three times weekly facilitate the implementation of all interventions.
Patient adherence may be challenging; in one report, patients missed an average of 2.5 hours weekly of rehabilitation.72 Patient adherence to the intervention is described at discharge by the patient, who will document participation in targeted education elements using a checklist. However, a 2016 study found that the most important factor facilitating participation in clinical studies was the possibility of learning more about SCI and health, which is a clear potential in the intervention study.73 A review by Van Wyk et al emphasises that patient education is an important part of the interdisciplinary rehabilitation of people with SCI and recommend an individualised approach and the use of different settings in which the patient can receive the education.29
Supplementary Material
Footnotes
Contributors: NJH has been primarily responsible for writing the protocol. TM (head supervisor), FB-S and LHS (cosupervisors) have all contributed to the development of the protocol and study design, as well as feedback, supervision and contributions to the text writing. LA has read and commented on several of the protocol drafts and contributed ideas for ensuring adherence of participants during the intervention. LTD has, in particular, contributed critical insights into the clinical setting and workflows involved in the project and the initial development and writing of the protocol. All authors approved the final version of the manuscript.
Funding: This work was supported by a research programme, 'Centre for Integrated Rehabilitation of Cancer Patients (CIRE)—Neuro/Psychology,' conducted collaboratively by the University Hospitals Centre for Health CareHealthcare Research, University Hospital Copenhagen, Rigshospitalet, University College Copenhagen, Department of Nursing and Nutrition, and the NeuroScience Centre, Rigshospitalet.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The project is approved by the Committees on Health Research Ethics in the Capital Region of Denmark on 10.07.2018 (Journal-nr.: H-18018325).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Biering-Sorensen F. Rygmarvsskade-den moderne behandling. Ugeskrift for Læger 2001;163:2766–9. [PubMed] [Google Scholar]
- 2. Nash MS, Groah SL, Gater DR, et al. . Identification and management of cardiometabolic risk after spinal cord injury: clinical practice guideline for health care providers. Top Spinal Cord Inj Rehabil 2018;24:379–423. 10.1310/sci2404-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Møller T, Moser C, Adamsen L, et al. . Early warning and prevention of pneumonia in acute leukemia by patient education, spirometry, and positive expiratory pressure: a randomized controlled trial. Am J Hematol 2016;91:271–6. 10.1002/ajh.24262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Møller T, Borregaard N, Tvede M, et al. . Patient education—a strategy for prevention of infections caused by permanent central venous catheters in patients with haematological malignancies: a randomized clinical trial. J Hosp Infect 2005;61:330–41. 10.1016/j.jhin.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 5. Rajan S, McNeely MJ, Warms C, et al. . Clinical assessment and management of obesity in individuals with spinal cord injury: a review. J Spinal Cord Med 2008;31:361–72. 10.1080/10790268.2008.11760738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wahman K, Nash MS, Lewis JE, et al. . Cardiovascular disease risk and the need for prevention after paraplegia determined by conventional multifactorial risk models: the Stockholm spinal cord injury study. J Rehabil Med 2011;43:237–42. 10.2340/16501977-0658 [DOI] [PubMed] [Google Scholar]
- 7. Libin A, Tinsley E, Nash M, et al. . Cardiometabolic risk clustering in spinal cord injury: results of exploratory factor analysis. Top Spinal Cord Inj Rehabil 2013;19:183–94. 10.1310/sci1903-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nash MS, Tractenberg RE, Mendez AJ, et al. . Cardiometabolic syndrome in people with spinal cord injury/disease: guideline-derived and nonguideline risk components in a pooled sample. Arch Phys Med Rehabil 2016;97:1696–705. 10.1016/j.apmr.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 9. Felleiter P, Krebs J, Haeberli Y, et al. . Post-Traumatic changes in energy expenditure and body composition in patients with acute spinal cord injury. J Rehabil Med 2017;49:579–84. 10.2340/16501977-2244 [DOI] [PubMed] [Google Scholar]
- 10. de Groot S, Post MWM, Postma K, et al. . Prospective analysis of body mass index during and up to 5 years after discharge from inpatient spinal cord injury rehabilitation. J Rehabil Med 2010;42:922–8. 10.2340/16501977-0605 [DOI] [PubMed] [Google Scholar]
- 11. Stenson KW, Deutsch A, Heinemann AW, et al. . Obesity and inpatient rehabilitation outcomes for patients with a traumatic spinal cord injury. Arch Phys Med Rehabil 2011;92:384–90. 10.1016/j.apmr.2010.07.235 [DOI] [PubMed] [Google Scholar]
- 12. Salem R, Bamer AM, Alschuler KN, et al. . Obesity and symptoms and quality of life indicators of individuals with disabilities. Disabil Health J 2014;7:124–30. 10.1016/j.dhjo.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, Henson S, Jackson AB, et al. . Obesity intervention in persons with spinal cord injury. Spinal Cord 2006;44:82–91. 10.1038/sj.sc.3101818 [DOI] [PubMed] [Google Scholar]
- 14. Nash MS, Kressler J. Model programs to address obesity and cardiometabolic disease: interventions for suboptimal nutrition and sedentary lifestyles. Arch Phys Med Rehabil 2016;97:S238–46. 10.1016/j.apmr.2016.05.026 [DOI] [PubMed] [Google Scholar]
- 15. Szlachcic Y, Adkins RH, Adal T, et al. . The effect of dietary intervention on lipid profiles in individuals with spinal cord injury. J Spinal Cord Med 2001;24:26–9. 10.1080/10790268.2001.11753551 [DOI] [PubMed] [Google Scholar]
- 16. Wong S, van Middendorp J, Belci M, et al. . Knowledge, attitudes and practices of medical staff towards obesity management in patients with spinal cord injuries: an international survey of four Western European countries. Spinal Cord 2015;53:24–31. 10.1038/sc.2014.168 [DOI] [PubMed] [Google Scholar]
- 17. Locatelli S, Gerber B, Goldstein B, et al. . Health care provider practices, barriers, and facilitators for weight management for individuals with spinal cord injuries and disorders. Top Spinal Cord Inj Rehabil 2014;20:329–37. 10.1310/sci2004-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sundhedsstyrelsen Fysisk aktivitet-håndbog Om forebyggelse OG behandling. Copenhagen, 2011. [Google Scholar]
- 19. Hicks AL, Martin Ginis KA, Pelletier CA, et al. . The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord 2011;49:1103–27. 10.1038/sc.2011.62 [DOI] [PubMed] [Google Scholar]
- 20. van der Scheer JW, Martin Ginis KA, Ditor DS, et al. . Effects of exercise on fitness and health of adults with spinal cord injury. Neurology 2017;89:736–45. 10.1212/WNL.0000000000004224 [DOI] [PubMed] [Google Scholar]
- 21. Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, et al. . Evidence-Based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord 2017. [DOI] [PubMed] [Google Scholar]
- 22. Rezende LS, Lima MB, Salvador EP. Interventions for promoting physical activity among individuals with spinal cord injury: a systematic review. J Phys Act Health 2018;15:954–9. 10.1123/jpah.2018-0034 [DOI] [PubMed] [Google Scholar]
- 23. Nash MS, Cowan RE, Kressler J. Evidence-Based and heuristic approaches for customization of care in cardiometabolic syndrome after spinal cord injury. J Spinal Cord Med 2012;35:278–92. 10.1179/2045772312Y.0000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin Ginis KA, Arbour-Nicitopoulos KP, Latimer AE, et al. . Leisure time physical activity in a population-based sample of people with spinal cord injury Part II: activity types, intensities, and durations. Arch Phys Med Rehabil 2010;91:729–33. 10.1016/j.apmr.2009.12.028 [DOI] [PubMed] [Google Scholar]
- 25. Nooijen CFJ, Post MWM, Spooren AL, et al. . Exercise self-efficacy and the relation with physical behavior and physical capacity in wheelchair-dependent persons with subacute spinal cord injury. J Neuroeng Rehabil 2015;12:103 10.1186/s12984-015-0099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greaves CJ, Sheppard KE, Abraham C, et al. . Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11:119 10.1186/1471-2458-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams TL, Ma JK, Martin Ginis KA. Participant experiences and perceptions of physical activity-enhancing interventions for people with physical impairments and mobility limitations: a meta-synthesis of qualitative research evidence. Health Psychol Rev 2017;11:179–96. 10.1080/17437199.2017.1299027 [DOI] [PubMed] [Google Scholar]
- 28. Nooijen CFJ, Stam HJ, Bergen MP, et al. . A behavioural intervention increases physical activity in people with subacute spinal cord injury: a randomised trial. J Physiother 2016;62:35–41. 10.1016/j.jphys.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 29. Nooijen CFJ, Stam HJ, Sluis T, et al. . A behavioral intervention promoting physical activity in people with subacute spinal cord injury: secondary effects on health, social participation and quality of life. Clin Rehabil 2017;31:772–80. 10.1177/0269215516657581 [DOI] [PubMed] [Google Scholar]
- 30. Chan A-W, Tetzlaff JM, Altman DG, et al. . Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krueger R, Casey M. Focus groups: a practical guide for applied research. Newbury Park, California: SAGE Publications, 2000. [Google Scholar]
- 32. Glaser B, Strauss A. The discovery of Grounded theory: strategies for qualitative research. New York: Aldine, 1980. [Google Scholar]
- 33. Møller T. Individualized supervised patient education: a key role in reinforcement of infection protection and outpatient management of acute leukemia (dissertation). University of Copenhagen, 2011. [Google Scholar]
- 34. van Wyk K, Backwell A, Townson A. A narrative literature review to direct spinal cord injury patient education programming. Top Spinal Cord Inj Rehabil 2015;21:49–60. 10.1310/sci2101-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lynch J, Cahalan R. The impact of spinal cord injury on the quality of life of primary family caregivers: a literature review. Spinal Cord 2017;55:964–78. 10.1038/sc.2017.56 [DOI] [PubMed] [Google Scholar]
- 36. Goosey-Tolfrey VL, Paulson TAW, Tolfrey K, et al. . Prediction of peak oxygen uptake from differentiated ratings of perceived exertion during wheelchair propulsion in trained wheelchair sportspersons. Eur J Appl Physiol 2014;114:1251–8. 10.1007/s00421-014-2850-9 [DOI] [PubMed] [Google Scholar]
- 37. Latimer-Cheung AE, Arbour-Nicitopoulos KP, Brawley LR, et al. . Developing physical activity interventions for adults with spinal cord injury. Part 2: motivational counseling and peer-mediated interventions for people intending to be active. Rehabil Psychol 2013;58:307–15. 10.1037/a0032816 [DOI] [PubMed] [Google Scholar]
- 38. Stacey D, Légaré F, Lewis K, et al. . Decision AIDS for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eerden S, Dekker R, Hettinga FJ. Maximal and submaximal aerobic tests for wheelchair-dependent persons with spinal cord injury: a systematic review to summarize and identify useful applications for clinical rehabilitation. Disabil Rehabil 2017:1–25. [DOI] [PubMed] [Google Scholar]
- 40. Billinger SA, Tseng BY, Kluding PM. Modified total-body recumbent stepper exercise test for assessing peak oxygen consumption in people with chronic stroke. Phys Ther 2008;88:1188–95. 10.2522/ptj.20080072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Billinger SA, Loudon JK, Gajewski BJ. Validity of a total body recumbent stepper exercise test to assess cardiorespiratory fitness. J Strength Cond Res 2008;22:1556–62. 10.1519/JSC.0b013e3181739dd7 [DOI] [PubMed] [Google Scholar]
- 42. DiPiro ND, Embry AE, Fritz SL, et al. . Effects of aerobic exercise training on fitness and walking-related outcomes in ambulatory individuals with chronic incomplete spinal cord injury. Spinal Cord 2016;54:675–81. 10.1038/sc.2015.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nightingale TE, Walhin JP, Thompson D, et al. . Predicting physical activity energy expenditure in wheelchair users with a multisensor device. BMJ Open Sport Exerc Med 2015;1 10.1136/bmjsem-2015-000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brage S, Brage N, Franks PW, et al. . Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. J Appl Physiol 2004;96:343–51. 10.1152/japplphysiol.00703.2003 [DOI] [PubMed] [Google Scholar]
- 45. Compher C, Frankenfield D, Keim N, et al. . Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 2006;106:881–903. 10.1016/j.jada.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 46. Fullmer S, Benson-Davies S, Earthman CP, et al. . Evidence analysis library review of best practices for performing indirect calorimetry in healthy and Non–Critically ill individuals. J Acad Nutr Diet 2015;115:1417–46. 10.1016/j.jand.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 47. Nightingale TE, Rouse PC, Thompson D, et al. . Measurement of physical activity and energy expenditure in wheelchair users: methods, considerations and future directions. Sports Med - Open 2017;3 10.1186/s40798-017-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Silveira SL, Ledoux TA, Robinson-Whelen S, et al. . Methods for classifying obesity in spinal cord injury: a review. Spinal Cord 2017;55:812–817. 10.1038/sc.2017.79 [DOI] [PubMed] [Google Scholar]
- 49. Piepoli MF, Hoes AW, Agewall S, et al. . 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016;23:Np1–96. 10.1177/2047487316653709 [DOI] [PubMed] [Google Scholar]
- 50. Bauman WA, Biering-Sørensen F, Krassioukov A. The International spinal cord injury endocrine and metabolic function basic data set. Spinal Cord 2011;49:1068–72. 10.1038/sc.2011.51 [DOI] [PubMed] [Google Scholar]
- 51. Bauman WA, Wecht JM, Biering-Sørensen F. International spinal cord injury endocrine and metabolic extended data set. Spinal Cord 2017;55:466–77. 10.1038/sc.2016.164 [DOI] [PubMed] [Google Scholar]
- 52. Catz A, Itzkovich M, Tesio L, et al. . A multicenter International study on the spinal cord independence measure, version III: Rasch psychometric validation. Spinal Cord 2007;45:275–91. 10.1038/sj.sc.3101960 [DOI] [PubMed] [Google Scholar]
- 53. Itzkovich M, Gelernter I, Biering-Sorensen F, et al. . The spinal cord independence measure (SCIM) version III: reliability and validity in a multi-center International study. Disabil Rehabil 2007;29:1926–33. 10.1080/09638280601046302 [DOI] [PubMed] [Google Scholar]
- 54. Anderson KD, Acuff ME, Arp BG, et al. . United States (US) multi-center study to assess the validity and reliability of the spinal cord independence measure (SCIM III). Spinal Cord 2011;49:880–5. 10.1038/sc.2011.20 [DOI] [PubMed] [Google Scholar]
- 55. Fekete C, Eriks-Hoogland I, Baumberger M, et al. . Development and validation of a self-report version of the spinal cord independence measure (SCIM III). Spinal Cord 2013;51:40–7. 10.1038/sc.2012.87 [DOI] [PubMed] [Google Scholar]
- 56. Kirshblum S, Waring W. Updates for the International standards for neurological classification of spinal cord injury. Phys Med Rehabil Clin N Am 2014;25:505–17. 10.1016/j.pmr.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 57. Kirshblum SC, Burns SP, Biering-Sorensen F, et al. . International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34:535–46. 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Poritz JMP, Mignogna J, Christie AJ, et al. . The patient health questionnaire depression screener in spinal cord injury. J Spinal Cord Med 2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Charlifue S, Post MW, Biering-Sørensen F, et al. . International spinal cord injury quality of life basic data set. Spinal Cord 2012;50:672–5. 10.1038/sc.2012.27 [DOI] [PubMed] [Google Scholar]
- 60. Post MWM, Adriaansen JJE, Charlifue S, et al. . Good validity of the International spinal cord injury quality of life basic data set. Spinal Cord 2016;54:314–8. 10.1038/sc.2015.99 [DOI] [PubMed] [Google Scholar]
- 61. Martin Ginis KA, Jörgensen S, Stapleton J. Exercise and sport for persons with spinal cord injury. PM&R 2012;4:894–900. 10.1016/j.pmrj.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 62. Kroll T, Kehn M, Ho P-S, et al. . The SCI exercise self-efficacy scale (ESES): development and psychometric properties. Int J Behav Nutr Phys Act 2007;4 10.1186/1479-5868-4-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Matthiessen J, Andersen LF, Barbieri HE, et al. . The Nordic monitoring system 2011–2014 status and development of diet, physical activity, smoking, alcohol and overweight. TemaNord, 2016. [Google Scholar]
- 64. Matthiessen J, Andersen L, Barbieri H, et al. . The Nordic monitoring of diet, physical activity, smoking, alcohol and overweight: 2011-2014. EJNFS 2017;7:128–30. 10.9734/EJNFS/2017/35072 [DOI] [Google Scholar]
- 65. Gater DR. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18:333–51. 10.1016/j.pmr.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 66. Liusuwan RA, Widman LM, Abresch RT, et al. . Behavioral intervention, exercise, and nutrition education to improve health and fitness (benefit) in adolescents with mobility impairment due to spinal cord dysfunction. J Spinal Cord Med 2007;30:S119–26. 10.1080/10790268.2007.11754615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. de Groot S, Dallmeijer AJ, Post MWM, et al. . The longitudinal relationship between lipid profile and physical capacity in persons with a recent spinal cord injury. Spinal Cord 2008;46:344–51. 10.1038/sj.sc.3102147 [DOI] [PubMed] [Google Scholar]
- 68. Haisma JA, Post MW, van der Woude LH, et al. . Functional independence and health-related functional status following spinal cord injury: a prospective study of the association with physical capacity. J Rehabil Med 2008;40:812–8. 10.2340/16501977-0258 [DOI] [PubMed] [Google Scholar]
- 69. Janssen TW, van Oers CA, Veeger HE, et al. . Relationship between physical strain during standardised ADL tasks and physical capacity in men with spinal cord injuries. Paraplegia 1994;32:844–59. [DOI] [PubMed] [Google Scholar]
- 70. van Koppenhagen CF, Post M, de Groot S, et al. . Longitudinal relationship between wheelchair exercise capacity and life satisfaction in patients with spinal cord injury: a cohort study in the Netherlands. J Spinal Cord Med 2014;37:328–37. 10.1179/2045772313Y.0000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Groot S, Bevers G, Post MWM, et al. . Effect and process evaluation of implementing standardized tests to monitor patients in spinal cord injury rehabilitation. Disabil Rehabil 2010;32:588–97. 10.3109/09638280903174414 [DOI] [PubMed] [Google Scholar]
- 72. Zanca JM, Dijkers MP, Hammond FM, et al. . Pain and its impact on inpatient rehabilitation for acute traumatic spinal cord injury: analysis of observational data collected in the SCIRehab study. Arch Phys Med Rehabil 2013;94:S137–44. 10.1016/j.apmr.2012.10.035 [DOI] [PubMed] [Google Scholar]
- 73. Anderson KD, Cowan RE, Horsewell J. Facilitators and barriers to spinal cord injury clinical trial participation: multi-national perspective of people living with spinal cord injury. J Neurotrauma 2016;33:493–9. 10.1089/neu.2015.4064 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-030310supp001.pdf (50.4KB, pdf)