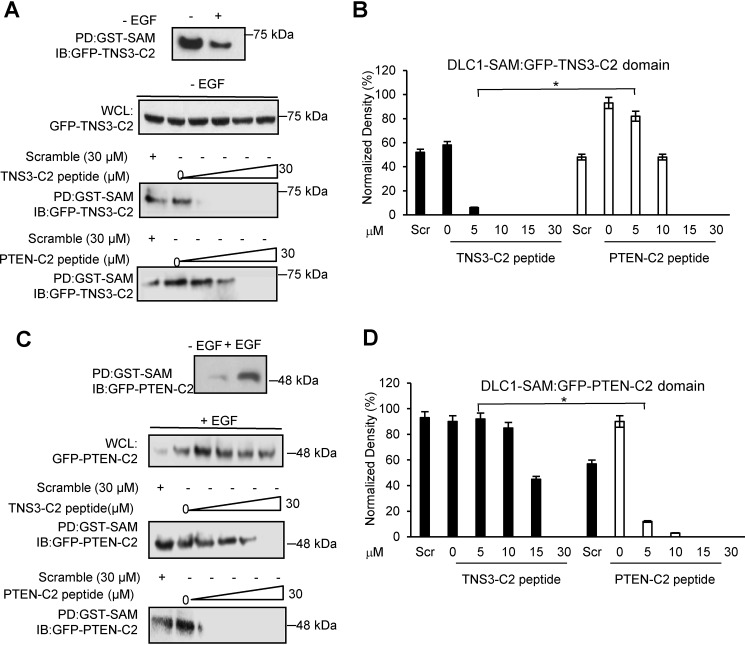
Figure 3.
The C2 peptides disrupted the DLC1 SAM-PTEN/TNS3-C2 domain-domain interactions in cells. A and B, effect of the C2 peptides on DLC1-SAM binding to the TNS3-C2 domain in HEK293DLC1 cells without EGF stimulation. The TNS3 C2 domain bound more tightly to the DLC1 SAM domain in the absence of EGF stimulation; accordingly, the TNS3-C2–derived peptide inhibited this interaction more effectively than the PTEN C2–derived peptide. Quantification of the Western blots indicates a significantly different effect between the two C2 peptides at 5 μm (n = 3; *, p < 0.01, Student's t test). C and D, effect of the C2 peptides on DLC1-SAM binding to the PTEN C2 domain in HEK293 cells in the presence of EGF. The PTEN-C2 domain bound to the SAM domain more tightly with EGF stimulation (for 30 min). Accordingly, the PTEN C2–derived peptide blocked this interaction more effectively than the TNS3 C2–derived peptide in HEK293 cells with EGF. Quantification of Western blots (D) indicates a significantly different effect between the two C2 peptides at 5 μm peptide (n = 3; *, p < 0.01, Student's t test). Scr: scrambled TSN3-C2 peptide control, applied at 30 μm.