Figure 5.
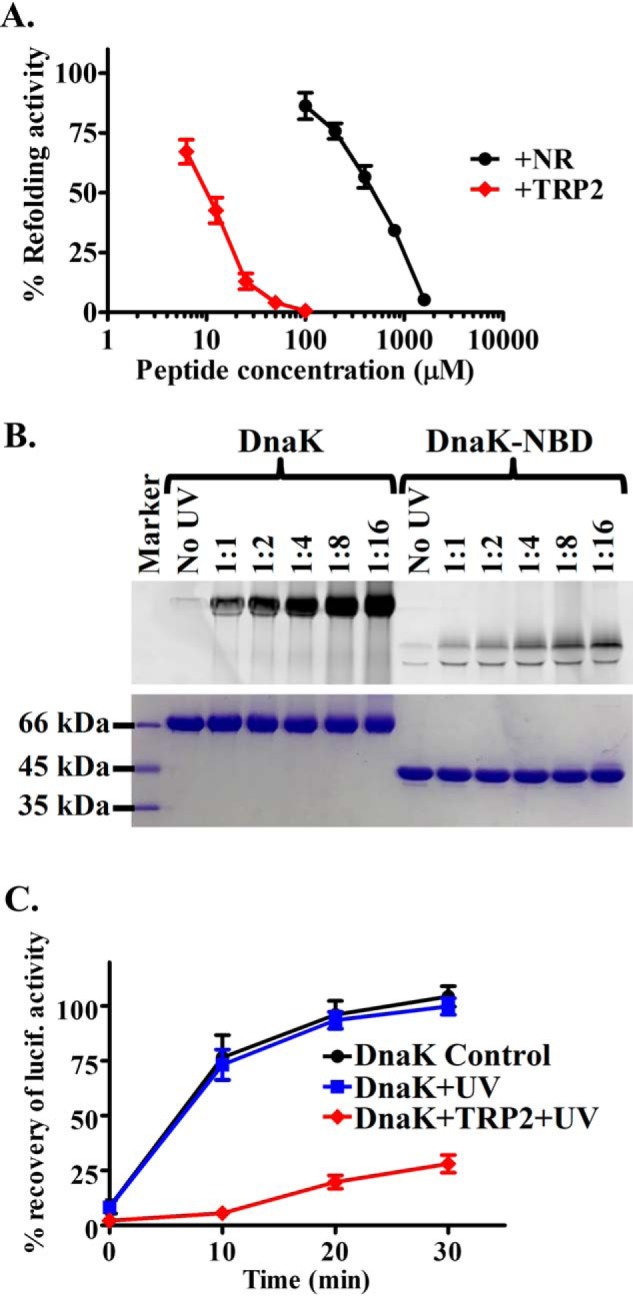
The new second peptide-binding site is essential for DnaK's chaperone activity. A, the TRP2 peptide inhibits the refolding activity of DnaK even more efficiently than the NR peptide. The refolding activities of DnaK were determined in the presence of the indicated concentrations of peptides. The refolding activity of DnaK in the absence of peptide was set as 100%. B, the TRP2-Bpa peptide was efficiently cross-linked to DnaK protein. DnaK protein was incubated with the TRP2-Bpa peptide at the indicated molar ratios and treated with UV light on ice. The isolated NBD of DnaK was used as a negative control. After SDS-PAGE, the cross-linked DnaK-peptide complex was visualized by fluorescence scan (top) and Coomassie Blue staining (bottom), respectively. C, the DnaK protein cross-linked with the TRP2-Bpa peptide has a compromised chaperone activity in refolding heat-denatured luciferase. The DnaK protein with only UV treatment was used as a control. The percentage of recovery of luciferase activity was calculated by setting the native luciferase activity as 100%. Error bars, S.E.
