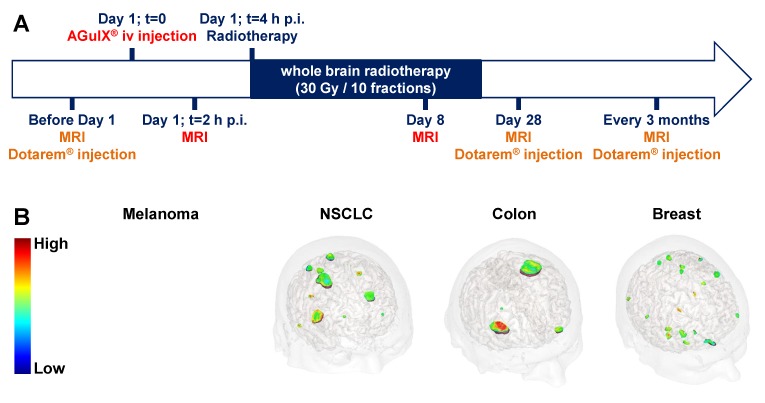
Figure 6.
Phase Ib clinical trial assessing AGuIX® NP radiosensitization. A. Protocol for the clinical trial of phase Ib (NCT03308604, NANORAD) to assess radiosensitization of multiple brain metastases using AGuIX® NP. This first-in-man clinical trial aims at studying the tolerance of AGuIX® NP iv administration in combination with whole brain radiation therapy and determining the recommended dose of AGuIX® NP for phase II clinical trial. B. 3D MRI from patients included in the NANORAD clinical trial obtained 2 h after iv injection of AGuIX® NPs. The brain metastases stemming from the four different types of primary cancers (melanoma, non-small cell lung cancer (NSCLC), colon cancer and breast cancer) were targeted by the AGuIX® NPs while no enhancement of the MRI signal was observed in healthy tissues. The patients were treated with 15 to 100 mg/kg of AGuIX® NPs (dose escalation). For the brought forward MRI data, the patients with melanoma, NSCLC, colon cancer and breast cancer received 15, 50, 50 and 75 mg/kg doses of AGuIX® NP respectively. Adapted with permission from ref 30, copyright 2019 BIR Publications.