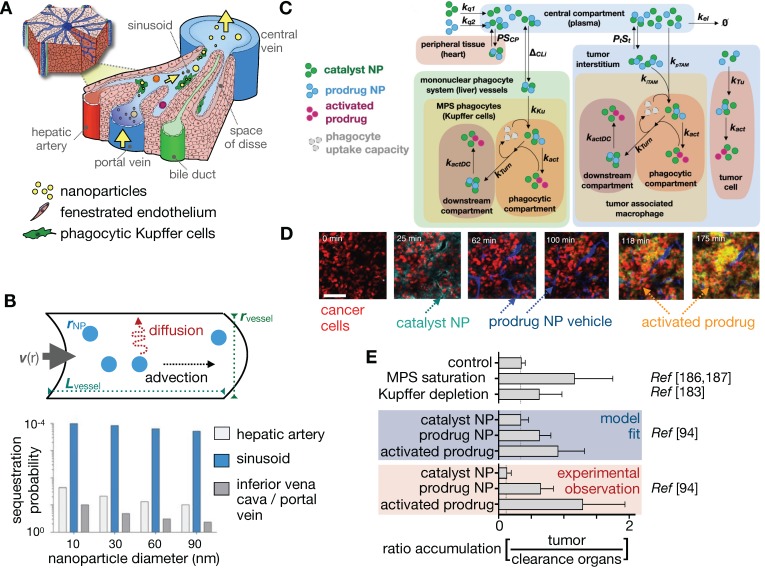
Figure 11.
Understanding and manipulating NP clearance by the mononuclear phagocytic system (MPS). (A) Schematic depicting NP dynamics in the liver parenchyma, with Kupffer cells phagocytosing NPs within the sinusoid. (B) Slow sinusoidal blood flow favors diffusive transport, long residence time near phagocytes and corresponding high cellular uptake compared to faster flowing vessels. In vitro microfluidics helped support this finding (Adapted with permission from 128, copyright 2016 Springer Nature). (C-E) A multi-compartmental model was used to evaluate the balance of systemic NP clearance by the MPS vs. NP uptake in TAMs and cancer cells. In particular, the model evaluated how a two-component NP system comprising a prodrug-encapsulated NP, and a second catalyst NP that activated the prodrug, could combine to yield more selective drug activation in the tumor. (D) Modeling in C was guided by IVM, represented here by time-lapse imaging of an initial catalyst NP, subsequent administration of a prodrug NP in the same subject, and finally followed by activation of the prodrug in extravascular tumor tissue (Adapted with permission from 94, copyright 2018 ACS Publications). (E) Comparison of different MPS evasion strategies in enabling selective NP accumulation in the tumor. MPS saturation using pre-treatment liposomes 186, 187, Kupffer cell depletion 183, and prodrug-NP/catalyst-NP administration 94 all showed improved tumor NP delivery compared to controls. In the case of the prodrug strategy, experimental results matched the fit of the computational model in C, and further analysis revealed putative mechanisms explaining the enhanced selectivity in delivery.