Abstract
Patient: Male, 78-year-old
Final Diagnosis: Presacral neuroendocrine tumors
Symptoms: Asymptomatic
Medication: —
Clinical Procedure: Peptide receptor radionuclide therapy
Specialty: Nuclear Medicine
Objective:
Rare disease
Background:
Primary neuroendocrine tumors (NETs) in the retroperitoneal space are extremely rare. We report the case of a patient diagnosed with primary presacral NET in the retroperitoneum that was initially suspected to be hepatic metastasis, who was followed up for more than 8 years.
Case Report:
A 78-year-old man was referred to our hospital for the treatment of a hepatic mass. Following resection, the patient was diagnosed with a grade 2 well-differentiated NET. A thorough evaluation to identify the primary tumor detected small well-demarcated presacral nodules on In-111 octreotide single-photon emission tomography/computed tomography (SPECT/CT). Metastases to other locations were not observed. Presacral nodules were difficult to remove using the surgical approach; therefore, we decided to follow up closely. After 4 years, the patient was diagnosed with recurrent hepatic metastasis and peritoneal seeding. Although combination therapy of everolimus and octreotide long-acting repeatable was administered, it was discontinued owing to disease progression. Baseline Ga-68 DOTATOC positron emission tomography-computed tomography revealed adequate avidity for the lesions observed on SPECT/CT; therefore, 5 cycles of peptide receptor radionuclide therapy (PRRT) were administered, after which stable disease was maintained.
Conclusions:
We identified an extremely rare primary retroperitoneal NET on In-111 octreotide SPECT/CT. During long-term follow-up, although the patient presented with recurrent hepatic metastases and peritoneal seeding, PRRT was successful in stabilizing the disease.
MeSH Keywords: Diagnostic Techniques, Radioisotope; Neuroendocrine Tumors; Positron-Emission Tomography; Radionuclide Imaging; Retroperitoneal Neoplasms; Tomography, Emission-Computed, Single-Photon
Background
Neuroendocrine tumors (NETs) are a heterogeneous group of malignant tumors arising from enterochromaffin cells, with varying clinical manifestations. NETs can occur in almost any organ, but are mainly observed in the gastroenteropancreatic system (70%), respiratory system (25%), and other primary sites (5%) [1]. As primary NETs in the retroperitoneal space are extremely rare, preoperative diagnosis is very difficult because of the indolent tumor characteristics and complex anatomy of the location in which the tumors occur [2]. Presacral well-differentiated NETs (WDNETs) in the retroperitoneum can occur directly or owing to metastasis from rectal carcinoids [2] or from primary presacral neoplasms. Small primary NETs are known to cause large hepatic metastases [3,4]. However, metastatic NETs are often observed in the liver, because the entire systemic blood supply passes through the liver, making it a prime target for metastatic disease [5].
The 2017 World Health Organization (WHO) classification divides neuroendocrine neoplasms (NENs) into 2 different groups: well-differentiated, low-proliferating NENs such as NETs or carcinoids); and poorly differentiated, highly proliferating NENs such as small- or large-cell neuroendocrine carcinomas (NECs) [1,6]. This classification, which is critical for assessing the tumor grade and the possible disease prognosis [7], is based on the histologic grade of the tumor considering the mitotic count and Ki-67 labeling index. When the Ki-67 index is low (<3%) and the mitotic count is <2 per 10 high-power fields (HPFs), the tumor is classified as a G1 NET. For G2 NETs, the Ki-67 index is 3–20% and the mitotic count is 2–20 per 10 HPFs. G3 NETs have a Ki-67 index >20% and a mitotic count of >20 per 10 HPFs [6]. The staging system for NENs has not yet been well-established. Herein, on the basis of this classification, we report a case of a G2 WDNET with hepatic metastasis arising from the presacral space, along with a literature review.
Case Report
A 78-year-old man was referred to our hospital for treatment of a left liver mass that was suspected to be a carcinoma, mostly hepatocellular carcinoma, after histological examination at another hospital. He was undergoing treatment for diabetes, which was well controlled, and had no history of smoking or consumption of alcohol. He also had no history of liver disease, including hepatitis and cirrhosis. Physical examination findings were unremarkable. The results of laboratory tests were within normal limits, including the levels of tumor markers such as alpha-fetoprotein, carbohydrate antigen 19-9, protein induced by vitamin K absence or antagonist II, and carcinoembryonic antigen. Abdominopelvic computed tomography (APCT) and gadolinium-enhanced magnetic resonance (MRI) scans revealed a round heterogeneous mass measuring 3 cm in the left lobe of the liver. The patient underwent laparoscopic left lateral sectionectomy. On histopathologic examination, tumor cells with abundant cytoplasm and vesicular nuclei chromatin were observed in a trabecular pattern. Immunohistochemical (IHC) staining and molecular studies of tumor cells revealed focal positivity for chromogranin, synaptophysin, and CD56. The mitotic count was 8 per 10 HPFs and the Ki-67 index was 6.6%. Thus, according to the 2017 WHO classification, the tumor was diagnosed as a G2 WDNET. Comprehensive tests were then performed to determine the primary site and the differential diagnosis of metastatic NETs. IHC staining revealed negative results for cytokeratin 7 (CK-7), thyroid transcription factor-1 (TTF-1), and CDX2. Chest CT, F-18 fludeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT), gastroduodenoendoscopy, and colonoscopy were unable to determine the primary tumor site; however, In-111 octreotide scans revealed 2 octreotide-avid nodules in the left side of the presacral retroperitoneum (Figure 1A, 1B). Corresponding heterogeneously enhanced nodules measuring 2.1×1.7 cm and 1.0×0.9 cm were observed in the presacral area on APCT (Figure 1C). No other metastases were found, and the presacral nodules were difficult to consider for surgery; hence, we decided to follow up closely. Approximately 4 years later, there was no significant change in the presacral lesions, but multiple new lesions were observed in the liver (Figure 2A, 2B). Ultrasound-guided biopsy was performed for the new lesions in the liver, which were subsequently diagnosed as G2 WDNETs. Peritoneal seeding was confirmed on the follow-up In-111 octreotide scan (Figure 2C). Metastatic NETs including hepatic and peritoneal seeding worsened and were accompanied by intractable diarrhea. The advanced and metastatic NETs were treated using a combination therapy of octreotide long-acting repeatable (LAR) repeatedly administered intramuscularly at a dose of 20 mg and everolimus 10 mg/day, which is an oral mammalian target of rapamycin (mTOR) inhibitor. Everolimus was administered for 6 weeks, but treatment was discontinued owing to the occurrence of grade 3 stomatitis. Although a transient treatment response was observed after octreotide LAR treatment for 10 months and everolimus re-treatment for 8 weeks, the presacral mass and metastatic lesions in the liver and peritoneum showed progression on radiologic examinations. Therefore, NETs were considered to be refractory to the combination treatment with octreotide LAR and everolimus, and the treatment was discontinued. Owing to the failure of other systemic treatments, the progressive NETs were treated with pazopanib (800 mg/day, an oral multi-kinase inhibitor). Baseline Ga-68 DOTATOC PET-CT was performed before the scheduled peptide receptor radionuclide therapy (PRRT) at another hospital because PRRT is not approved in Korea (Figure 3). After 5 cycles of 8.0 GBq PRRT, Lu-177 DOTATATE performed after an 8-week interval revealed that the presacral and metastatic lesions showed no significant changes (Figure 4A, 4B). The patient was then closely followed up.
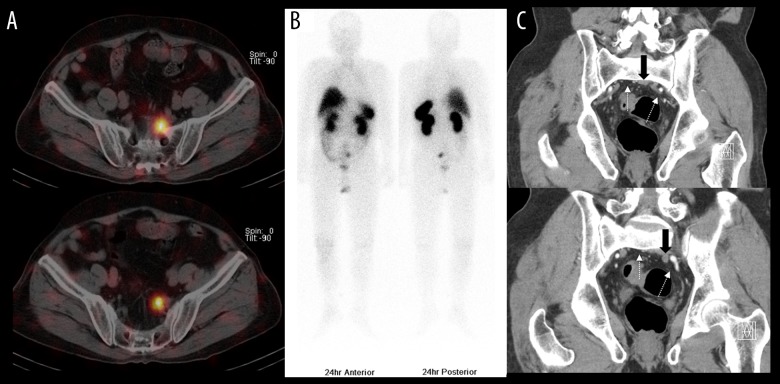
Figure 1.
(A) Axial In-111 octreotide SPECT/CT findings. There were 2 well-demarcated small round nodules with In-111 octreotide uptake in the presacral area on 24-h In-111 octreotide scans. (B) Anterior-posterior whole-body In-111 octreotide scan findings. There were no other abnormal In-111 octreotide-avid lesions in the rest of the body. Normal physiologic uptake was noted in the liver, spleen, kidneys, bowels, and bladder. (C) Coronal abdominopelvic enhanced computed tomography findings: Heterogeneously enhanced nodules of 2.1×1.7 cm and 1.0×0.9 cm (solid black arrow) were noted abutting the left internal iliac vessels and presacral fascia (white dotted arrow).
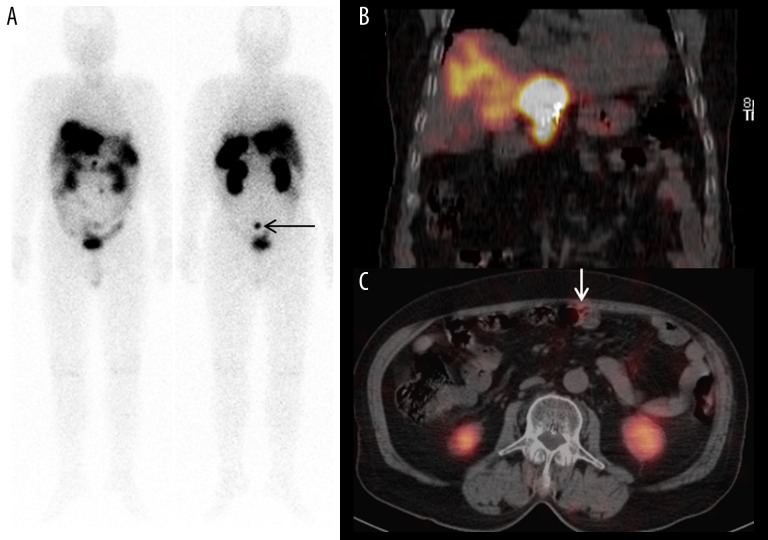
Figure 2.
(A, B) Anterior-posterior whole-body In-111 octreotide scan findings and coronal In-111 octreotide SPECT/CT findings. Multiple intense In-111 octreotide-avid hepatic masses recurred in the rest of the liver. Presacral nodules were also visualized, with no significant interval change compared with that observed on the In-111 octreotide scan 4 years previously (black arrow in (A)). (C) Axial In-111 octreotide SPECT/CT findings. Newly developed peritoneal seeding nodules with In-111 octreotide uptake were also noted (white arrow).
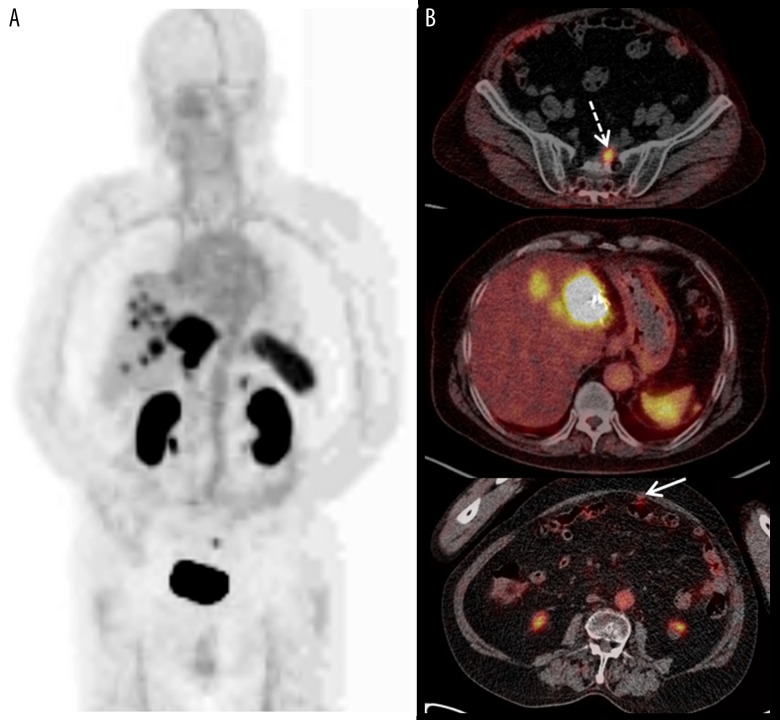
Figure 3.
(A, B) Maximal intensive projection/axial Ga-68 DOTATOC PET/CT. Multiple nodules with Ga-68 DOTATOC uptake were noted in the residual liver. A presacral Ga-68 DOTATOC-avid nodule was also noted (white dotted arrow); however, there was no Ga-68 DOTATOC uptake in the smaller presacral nodule. Multiple peritoneal nodules with Ga-68 DOTATOC uptake (white arrow) were also observed in the corresponded area on the In-111 octreotide scan.
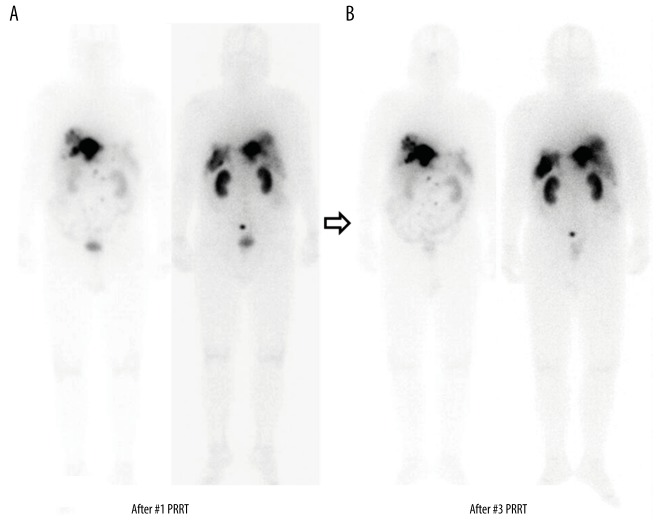
Figure 4.
(A, B) Anterior-posterior whole-body scan findings after the first and third cycles of Lu-177 DOTATATE. After the first and third cycles of 8.0 GBq Lu-177 DOTATATE therapies, whole-body scans were obtained after 24 h. There was no significant interval change in the multiple nodules that showed Lu-177 DOTATATE uptake in the retroperitoneum, liver, and peritoneum.
Discussion
This is a report of a rare primary retroperitoneal NET that initially presented as a hepatic mass that was otherwise missed on conventional imaging modalities. The presacral region in the retroperitoneum is usually a potential space and is clinically important; it is composed of complex anatomical structures, such as the axial muscles, lumbosacral nerve trunk, sacral plexus, iliac vessels, and pelvic soft tissues, as well as multiple embryological remnants [8].
The diagnosis and identification of primary hepatic NETs are difficult and controversial because of the following reasons. (1) The liver does not contain neuroendocrine cells. (2) Metastasis to the liver is the most common, irrespective of the NET grade and it is synchronous with other metastases in 45–95% of cases [7]; in addition, small primary NETs are known to cause large metastases in the liver [5]. (3) There have been no morphologic or IHC markers to definitely rule out the extra-hepatic origin [5]. (4) A thorough examination of other suspected primary sites is needed to confirm the primary site of metastases to the liver. Histopathological examinations including IHC (e.g., chromogranin A, synaptophysin, and CD56) are important for diagnosing NETs. In our case, IHC staining was performed to identify primary sites, and hepatic metastatic NETs were negative for TTF-1 (a marker for a differentiated meta-static tumor originating from the foregut NET), CK-7 (a marker for cholangiocarcinoma), and CDX2 (a highly sensitive and specific marker for adenocarcinomas of intestinal origin) [8]. No lesions were suspected to be primary NET from the stomach to the rectum on endoscopic evaluation.
Imaging studies play an essential role in the detection and localization of primary and/or metastatic NETs [9]. CT and/or MRI have excellent sensitivity and detection rates, both of which are approximately 80% (76–100% for CT and 67–100% for MRI), because primary and metastatic NETs are usually well enhanced after the intravenous injection of contrast agents [9]. In addition, CT is useful for the detection of primary site tumors when the location of the primary NETs is unknown, and MRI easily reveals hepatic metastasis of NETs and primary pancreatic NETs [9]. Our patient also showed no abnormal lesions on APCT and no definite space-occupying lesion in the pancreas on abdominal MRI scan. F-18 FDG PET/CT is used to detect malignant tumors among many different tumor types. Unfortunately, low-to-intermediate grade NETs are relatively metabolically inactive, while high-grade NETs demonstrate FDG avidity. The present case was a G2 WDNET; therefore, all the hepatic and retroperitoneal lesions found in our patient showed isometabolic activity, while FDG avidity was not definitely observed in the small peritoneal seedings [10].
Somatostatin is an endogenous peptide secreted by neuroendocrine cells [10]. The surfaces of WDNETs show high expression of somatostatin receptors (SSRs), and the interactions between radiolabeled somatostatin analogues (SSAs) and SSRs on tumor cells make them targets for treatment and imaging [10,11]. There are 2 types of SSR-based imaging available: In-111 octreotide scan and Ga-68 DOTATOC imaging. In-111 octreotide is the first-line scintigraphic agent and is the most widely studied agent; In-111 octreotide imaging is often used in combination with SPECT/CT [10,12]. In-111 octreotide scanning has a sensitivity of less than 60%, although it has excellent specificity among functional imaging studies [11]. Nevertheless, the ability of In-111 octreotide scan to detect the primary tumor is determined by the tumor size and not the expression of SSRs [10]. Since the development of Ga-68 SSAs for PET/CT, molecular imaging studies for the detection of NET have evolved rapidly over the last 15 years [11]. The sensitivity of Ga-68 SSA PET-CT is above 90% and the specificity is 92–98%, which is better than those of CT, MRI, and In-111 octreotide scanning. Therefore, In-111 octreotide scanning is useful to detect small tumors in the lymph nodes or bones, as well as unknown primary NETs [11,13,14]. The indications for SSR-based imaging include the detection of primary NETs and their metastases, tumor staging, surveillance for recurrence, and the selection of patients for PRRT. Our patient had liver and peritoneal metastases with presacral nodules on pre-treatment Ga-68 DOTATOC PET/CT before PRRT. Using this imaging modality, the most likely primary tumor location was the presacral space based on the results of IHC staining, In-111 octreotide scan, and other conventional imaging studies. However, the primary tumor location had not been confirmed on histological examination because the possible primary NETs in the presacral area could not be excised owing to the difficulty in using the surgical approach. The tumor in the present case could not be ruled out as metastasis from a missed small primary tumor at another location. Neither biochemical nor radiologic investigations, which are commonly used for the evaluation of metastatic NETs, were useful in ruling out this possibility.
Presacral NETs are usually asymptomatic and cause symptoms associated with their mass effect, such as low back pain and constipation [8]. Presacral NETs usually do not cause symptoms of carcinoid syndrome, such as flushing, sweating, and hypertension; however, in the current case, the patient developed intractable diarrhea, which indicates carcinoid syndrome, usually observed in patients with advanced and metastatic NETs. The first-line systemic treatment for patients with advanced or metastatic NETs is usually a combination of SSAs for controlling the tumor growth and hormonal secretion, and everolimus [15–17]. The combination therapy of SSAs and mTOR inhibitors can improve the progression-free survival (PFS) but not the overall survival [15]. Pazopanib is an oral multi-kinase inhibitor that is a treatment option for advanced and meta-static NETs because it increases the PFS of patients who did not respond to other systemic treatments including mTOR inhibitors and other multitargeted agents [18].
PRRT is another treatment modality that includes the use of radiolabeled SSAs for patients with advanced or metastatic NETs refractory to octreotide LAR treatment. It is associated with markedly longer PFS and better clinical outcomes [16,17]. Lu-177 is a β-emitter that has a higher range and energy than other radionuclides as well as higher emission of γ-rays, thereby making it useful for monitoring tumor response. Lu-177 DOTATATE results in a significantly higher response rate and an improvement in the quality of life, with a 79% reduction in the risk of progression or death [11,17]. In our patient, stable disease was maintained after administering 5 cycles of PRRT.
Conclusions
Clinicians and radiologists need to consider the diagnostic difficulty associated with small and asymptomatic primary presacral NETs, as well as its rarity. Our patient initially presented with a hepatic mass; however, using In-111 octreotide SPECT/CT, we successfully diagnosed small primary presacral NETs, which was otherwise missed on conventional imaging modalities. During the long-term follow-up, although the disease progressed with recurrent hepatic metastases and peritoneal seeding refractory to the systemic treatment, stable disease was maintained through PRRT. As shown in this case, the long-term progressive clinical course of presacral NETs could help understand these rare diseases.
Footnotes
Ethics approval
This study was conducted in compliance with the Institutional Review Board (IRB) regulations (approval ID: HPIRB 2019-09-003) and the Declaration of Helsinki.
Conflict of interest
None.
References:
- 1.Klöppel G. Neuroendocrine neoplasms: Dichotomy, origin and classifications. Visc Med. 2017;33(5):324–30. doi: 10.1159/000481390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Rosa S, Boni L, Finzi G, et al. Ghrelin-producing well-differentiated neuroendocrine tumor (carcinoid) of tailgut cyst. Morphological, immunohistochemical, ultrastructural, and RT-PCR study of a case and review of the literature. Endocr Pathol. 2010;21(3):190–98. doi: 10.1007/s12022-010-9127-6. [DOI] [PubMed] [Google Scholar]
- 3.Howell DL, O’Dorisio MS. Management of neuroendocrine tumors in children, adolescents, and young adults. J Pediatr Hematol Oncol. 2012;34:S64–68. doi: 10.1097/MPH.0b013e31824e3885. [DOI] [PubMed] [Google Scholar]
- 4.Hubalewska-Dydejczyk A, Trofimiuk M, Sowa-Staszczak A, et al. Neuroendocrine tumours of rare location. Endokrynol Pol. 2010;61(3):322–27. [PubMed] [Google Scholar]
- 5.Pastrian LG, Ruz-Caracuel I, Gonzalez RS. Giant primary neuroendocrine neoplasms of the liver: Report of 2 cases with molecular characterization. Int J Surg Pathol. 2019 doi: 10.1177/1066896919855764. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. World Health Organization; 2010. [Google Scholar]
- 7.Silveira F, Basile ML, Kuga FS, et al. Neuroendocrine tumors: An epidemiological study of 250 cases at a tertiary hospital. Rev Assoc Med Bras (1992) 2017;63(10):856–61. doi: 10.1590/1806-9282.63.10.856. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Lee NK, Kim S, et al. Primary presacral neuroendocrine tumor: A case report and review of MRI Findings. J Korean Soc Radiol. 2017;77(3):187–91. [Google Scholar]
- 9.Dehal A, Kim S, Ali A, Walbolt T. Primary epithelial neuroendocrine tumors of the retroperitoneum. Perm J. 2015;19(4):71–75. doi: 10.7812/TPP/15-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maxwell JE, Howe JR. Imaging in neuroendocrine tumors: An update for the clinician. Int J Endocr Oncol. 2015;2(2):159–68. doi: 10.2217/ije.14.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maqsood MH, Tameez Ud Din A, Khan AH. Neuroendocrine tumor therapy with Lutetium-177: A literature review. Cureus. 2019;11(1):e3986. doi: 10.7759/cureus.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smit Duijzentkunst DA, Kwekkeboom DJ, Bodei L. Somatostatin receptor 2-targeting compounds. J Nucl Med. 2017;58(Suppl. 2):54S–60S. doi: 10.2967/jnumed.117.191015. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosini V, Campana D, Nanni C, et al. Is (6)(8)Ga-DOTA-NOC PET/CT indicated in patients with clinical, biochemical or radiological suspicion of neuroendocrine tumour? Eur J Nucl Med Mol Imaging. 2012;39(8):1278–83. doi: 10.1007/s00259-012-2146-4. [DOI] [PubMed] [Google Scholar]
- 14.Fanti S, Ambrosini V, Tomassetti P, et al. Evaluation of unusual neuroendocrine tumours by means of 68Ga-DOTA-NOC PET. Biomed Pharmacother. 2008;62(10):667–71. doi: 10.1016/j.biopha.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Yau H, Kinaan M, Quinn SL, Moraitis AG. Octreotide long-acting repeatable in the treatment of neuroendocrine tumors: patient selection and perspectives. Biologics. 2017;11:115–22. doi: 10.2147/BTT.S108818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Khaldi M, Mesbah A, Dube P, et al. Neuroendocrine carcinoma arising in a tailgut cyst. Int J Surg Case Rep. 2018;49:91–95. doi: 10.1016/j.ijscr.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grande E, Capdevila J, Castellano D, et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE) Ann Oncol. 2015;26(9):1987–93. doi: 10.1093/annonc/mdv252. [DOI] [PubMed] [Google Scholar]