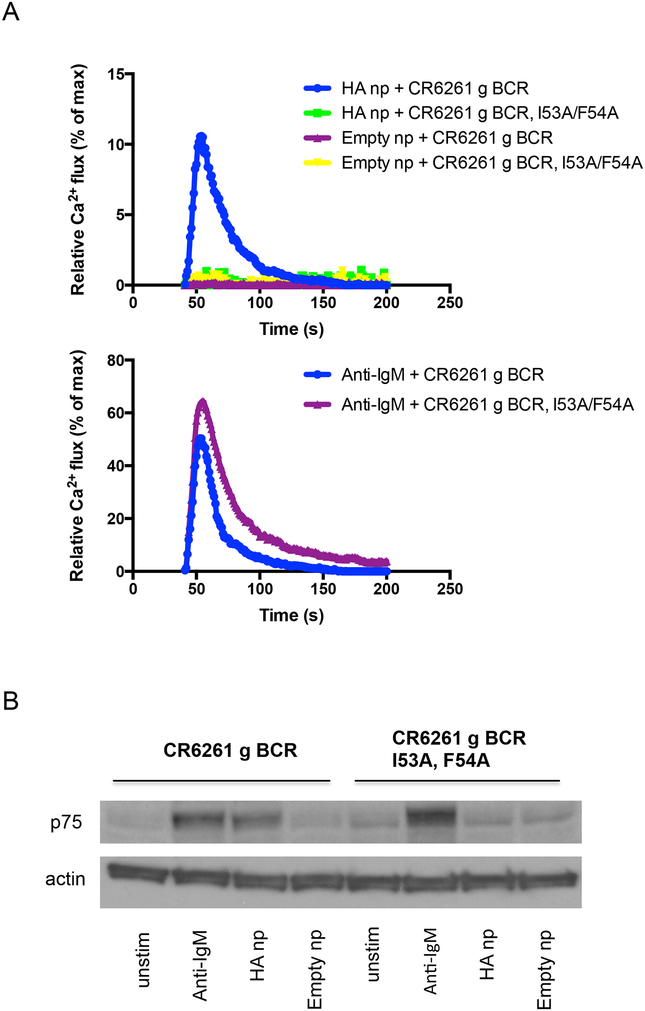
Figure 7.
BCR activation in response to multimerized candidate immunogen. For a kinetic readout, multimerized antigen is added to cells displaying BCR of interest that have been equilibrated with Fura Red, a ratiometric calcium-reporting fluorescent dye30. Calcium bound versus unbound emission is simultaneously measured following antigen addition. (A) CDRH2-dependent calcium flux through germline CR6261 BCR in response to HA ferritin nanoparticle (np) is presented. Negative controls include empty ferritin nanoparticle and I53A/F54A germline CR6261 BCR that is unable to engage the HA stem. This data is made relative to total membrane calcium flux as measured by incubation with the calcium ionophore ionomycin. When comparing different BCR receptors, it is also important to demonstrate that total receptor calcium flux, as measured via crosslinking by anti-IgM is comparable. Curves represent the average of 2–3 replicates. (B) BCR triggering can also be measured using an end point phosphorylation assay. In Ramos, p75 as measured by the antibody G410 pY serves as a reporter of this receptor output6,45,46. Western blotting for tyrosine phosphorylation following addition of multimerized antigen shows analogous activation of germline CR6261 BCR but not its I53A/F54A stem-binding mutant in response to HA np. As with the kinetic readout, empty ferritin nanoparticle does not induce signaling. Total receptor output is presented as tyrosine phosphorylation in response to anti-IgM, and actin levels are included as a loading control.