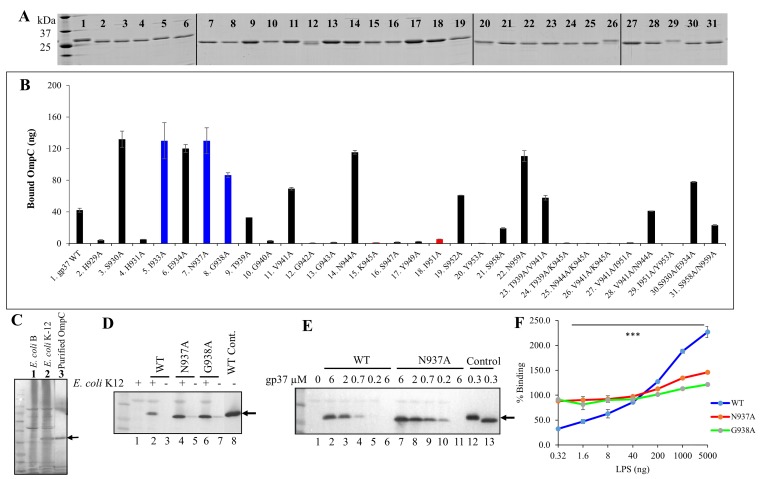
Fig 5. Modulation of LTF-receptor interactions.
(A) SDS-PAGE of purified mutant LTF needle proteins. The first lane on the left show molecular mass standards. A vertical line was added between images that were sliced and pasted. (B) Quantification of LTF needle-OmpC interaction by direct binding ELISA assay. Binding results that correlated with the in vivo genetic assays (Fig 4) are shown as black bars and those that did not correlate are shown either as blue bars (greater binding than WT needle) or red bars (poorer binding than WT needle). (C) Western blot with anti-OmpC antibody showing the presence of OmpC band in the E. coli K12 strain (lane 2), but not in the B strain (lane 1). The purified OmpC protein was included in lane 3 as a positive control. (D) Binding of WT and mutant LTF needle proteins to E. coli. Bound gp37 was detected by Western blotting using monoclonal anti-histag antibodies. Lane 1 is negative control in which the gp37 protein was omitted. Lanes 3, 5, and 7 represent negative controls where E. coli was omitted. Lane 8 is the positive control where purified WT gp37 was loaded into the well. (E) Dependence of protein concentration on the binding of WT and N937A gp37 proteins to E. coli K12 bacteria. Lane 1 is the negative control where the gp37 protein was omitted and lanes 6 and 11 are negative controls where E. coli was omitted. Arrows show the position of the gp37 band. (F) Binding of WT gp37, N937A, and G938A proteins to E. coli bacteria in the presence of increasing concentrations of purified E. coli B LPS.