Table 1.
In vitro activity of synthesized compounds on hTRPV1a
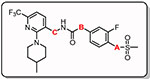 | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | Binding affinity Ki(nM) | Agonism EC50 (nM) | Antagonism Ki(ant) (nM) | |||
| 1 | –NH | CH–Me | CH2 | 7.90 | (±1.6) | NE | 2.22 | (±0.47) |
| 1S | –NH | (S)-CH–Me | CH2 | 2.95 | (±0.73) | NE | 1.26 | (±0.28) |
| 11 | –NH | CH–Me | (CH2)2 | 54 | (±13) | NE | 95 | (±26) |
| 16 | –CH2NH | CH–Me | CH2 | 230 | (±32) | NE | 27.6 | (±5.5) |
| 21 | –NH | NH | CH2 | 83 | (±27) | NE | 149 | (±50) |
| 22 | –NH | NH | (CH2)2 | 210 | (±46) | 29% | 49% | |
| 27 | –CH2NH | NH | CH2 | 6.77 | (±0.48) | NE | 12.5 | (±4.2) |
| 31 | –NH | NHCH2– | CH2 | 59 | (±12) | NE | 18.7 | (±6.1) |
| 32 | –NH | NHCH(Me)– | CH2 | 31.6 | (±3.4) | NE | 28.1 | (±8.5) |
| 34 | –CH2NH | NHCH2– | CH2 | 410 | (±27) | NE | 199 | (±50) |
| 41 | –NH | NH–Me | CH2 | 1160 | (±180) | NE | 59 | (±20) |
| 42 | –NH | NH–Ph(3-Me) | CH2 | 490 | (±120) | NE | 860b | (±290)b |
NE: no effect; values are the mean ± SEM of at least three experiments; where indicated, % represents the % agonism or antagonism at 3–30 μM.
Measured with 30 min pre-incubation before challenge with capsaicin; value with 15 min pre-incubation Ki(ant) = 2200 ± 560 nM.
