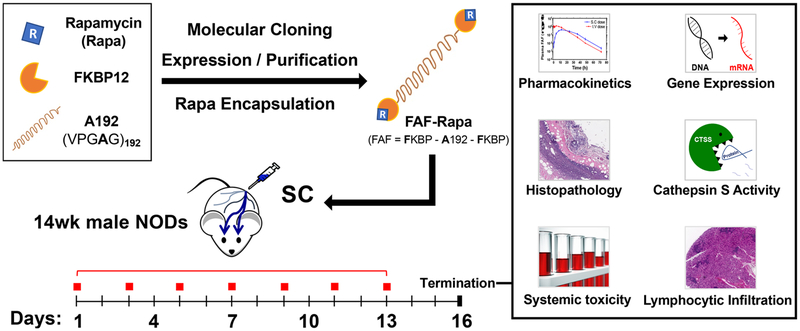
Figure 1.
Berunda polypeptides are humanized fusions of the FKBP12 protein that promote solvent-free, burst-free subcutaneous (SC) administration of Rapa to a murine model of autoimmune dacryoadenitis. Genes encoding the FK506-binding protein (12 kDa) were fused to each end of an ELP called A192 (73 kDa) to create a biheaded, biocompatible, and biodegradable drug carrier known as FAF. FAF was expressed via bacterial fermentation, purified at high yield by ELP-mediated purification, and used to solubilize Rapa. To explore the immunosuppressive properties of this formulation, FAF–Rapa was evaluated after SC injection to 14 week old male non-obese diabetic (NOD) mice every other day for 2 weeks. Male NOD mice of this age have developed autoimmune inflammation of the lacrimal gland (LG), also known as dacryoadenitis, leading to reduced tear production and dry eyes. At the termination of the study (Day 16), LG, tears, tissues, and serum were collected and analyzed using histology, gene expression, serum biochemistry, and the activity of a tear and tissue biomarker for SS known as cathepsin S.