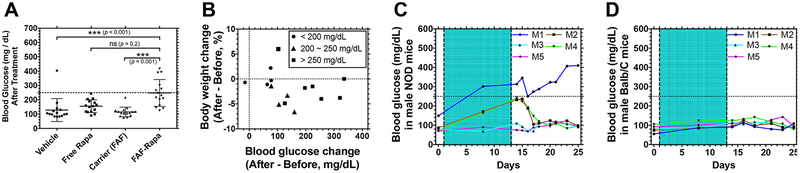
Figure 8.
FAF–Rapa induces temporary hyperglycemia in NOD mice that resolves after termination of the treatment. (A) At the conclusion of the study as described in Figure 1, blood glucose levels of individual mice were measured. The dotted line shows 250 mg/dL, which is a criterion for hyperglycemia. A Kruskal–Wallis nonparametric test was performed based on a statistical significance achieved by Rapa treatment (Vehicle + FAF vs free Rapa + FAF–Rapa, p < 0.001) and significant interaction between Rapa and FAF (p = 0.004) using two-way ANOVA (Mean ± SD, n = 15). Results of the Kruskal–Wallis nonparametric test are presented. (B) Each mouse treated with FAF–Rapa was further analyzed to correlate percent body weight change to blood glucose change, before and after the treatment. Mice with final blood glucose less than 200 mg/dL (circle), between 200 and 250 mg/dL (triangle), and above 250 mg/dL (square) were plotted. (C,D) In two additional studies, FAF–Rapa was administered as described in Figure 1 (shaded area) to either (C) male NOD mice (n = 5) or (D) male Balb/C mice (n = 5), and the blood glucose levels of individual mice (M1~M5) were monitored for 2 weeks after termination of the treatment.