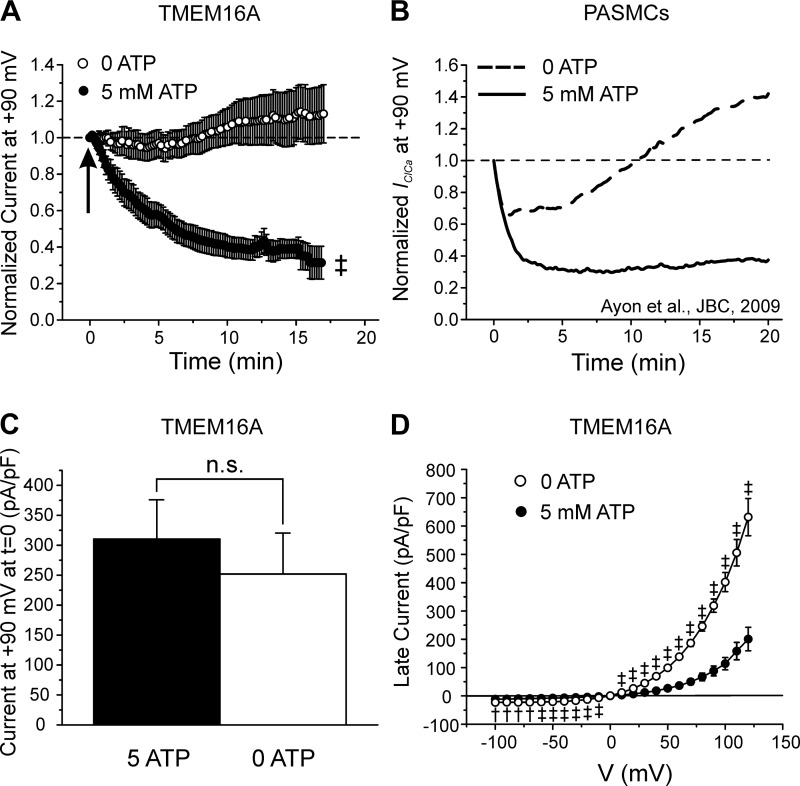
Fig. 3.
ATP dependence of TMEM16A-mediated Ca2+-activated Cl− currents (IClCa) and comparison with native IClCa. A: mean time courses of changes of normalized TMEM16A-induced IClCa measured at +90 mV in HEK-293 cells dialyzed with 0 (open circles; n = 14) or 5 mM ATP (closed circles; n = 26). Arrow indicates when current density was measured to compare the magnitude of the initial IClCa recorded at time = 0 in cells dialyzed with 0 or 5 mM ATP, for which mean data are reported in C. B: mean time courses of changes of native IClCa recorded in rabbit pulmonary artery smooth muscle cells (PASMCs) dialyzed with 0 (dashed line) or 5 mM ATP (solid line). [Plot was adapted from research originally published by our group in the Journal of Biological Chemistry (2), with permission. © the American Society for Biochemistry and Molecular Biology.] A and B emphasize the remarkable similarity in the response of TMEM16A and native IClCa to intracellular ATP. C: mean bar graph showing the magnitude of the initial late TMEM16A current (in pA/pF) recorded immediately after seal rupture (t = 0) in cells dialyzed with 5 (closed bar; n = 23) or 0 mM (open bar; n = 14) ATP. D: mean current-voltage (I-V) relationships for TMEM16A-induced IClCa recorded 20 min after seal rupture in HEK-293 cells dialyzed with 0 (open circles; n = 15) and 5 mM ATP (closed circles; n = 17). Both I-Vs displayed pronounced outward rectification and reversed around 0 mV, which is near the predicted equilibrium potential for Cl− in our conditions. For all panels: †P < 0.01; ‡P < 0.001; n.s., not significant.