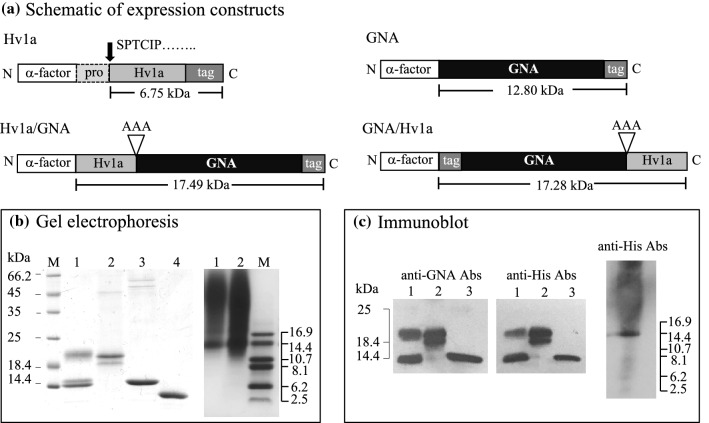
Fig. 1.
a Schematic of constructs encoding recombinant proteins produced in the yeast P. pastoris showing predicted molecular masses including tri-alanine linkers. The α-factor pre-pro-sequence directs expressed protein to the yeast secretory pathway enabling purification from fermented culture supernatants. Tag denotes the presence of a six-residue histidine sequence that allows recombinant protein purification by nickel affinity chromatography. b Separation of purified recombinant proteins by SDS-PAGE gels stained for total protein: left, lane 1 Hv1a/GNA, lane 2 GNA/Hv1a, lane 3 GNA and lane 4 Sigma GNA standard, right, lanes 1 and 2 Hv1a, c western analysis of recombinant proteins using anti-GNA and anti-His antibodies, approx. 300 ng total protein loaded in all lanes. Location of protein mass markers run on the same gel are depicted