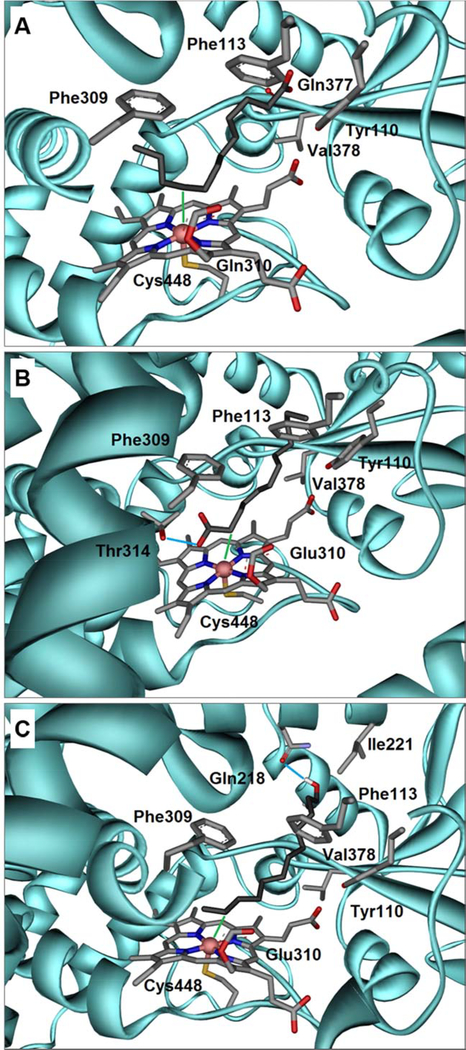
Figure 5.
(A) Docking of lauric acid 4, into the crystal structure of rabbit CYP4B1 demonstrates a U-shaped folding of the carbon chain inside the binding pocket. Here, the ω−3 position is closest to the Heme-Fe (3.04 Å) denoted as green line. The Heme moiety is covalently linked via the OE2 atom of glutamine 310 and the sulfur atom of cysteine 448 furthermore ligates the iron atom. The carboxylate group of C12 4 forms a hydrogen-bond with the backbone oxygen atom of glutamine 377. Also shown are the side chains of the residues forming close hydrophobic contacts; parts of the helical secondary structure were omitted for clarity. (B) A docked conformation of C12 4 showing an alternative binding pose in which the front positions (α, β, γ, δ) get close to the Heme-Fe, whereby the α-position is within 3.31 Å (green line). The carboxylate group of C12 4 forms a hydrogen-bond with the side chain of threonine 314 denoted as light blue line. (C) The obtained docking conformation of 1-dodecanol 10 shows that the terminal positions are preferably hydroxylated. Among those the ω−1 position is closest to the Heme-Fe (2.94 Å), indicated as green line. The hydroxyl group of C12-ol 10 forms a hydrogen-bond with the side chain of glutamine 218 denoted as light blue line. Color code for all Figures: grey = carbon; red = oxygen; blue = nitrogen; yellow = sulfur; coral pink = iron; cyan = CYP4B1 amino acid backbone. The respective pdb-files of the docked ligands in the corresponding reference frame are available as Supplementary Material.