Abstract
Acylated lysine residues represent major chemical modifications in proteins. We investigated the malonylation and propionylation of lysine residues (MalK, PropK) in the proteins of aging human lenses. Western blot results showed that the two modifications are present in human lens proteins. Liquid chromatography-mass spectrometry (LC-MS/MS) results showed 4–18 and 4–32 pmoles/mg protein of MalK and PropK, respectively, in human lens proteins with no apparent changes related to aging. Mass spectrometry results revealed that MalK- and PropK-modified lysine residues are present in all major crystallins, other cytosolic proteins, and membrane and cytoskeletal proteins of the lens. Several mitochondrial and cytosolic proteins in cultured human lens epithelial cells showed MalK and PropK modifications. Sirtuin 3 (SIRT3) and sirtuin 5 (SIRT5) were present in human lens epithelial and fiber cells. Moreover, lens epithelial cell lysate deacylated propionylated and malonylated lysozyme. The absence of SIRT3 and SIRT5 led to higher PropK and MalK levels in mouse lenses. Together, these data suggest that MalK and PropK are widespread modifications in lens and SIRT3 and SIRT5 could regulate their levels in lens epithelial cells.
Keywords: lens proteins, malonylation, propionylation, sirtuins, mass spectrometry
1. Introduction
Proteins of the eye lens have a negligible turnover rate and therefore accumulate posttranslational modifications (PTMs) throughout aging. PTMs, such as phosphorylation, carbamylation, methylation, advanced glycation, deamidation and oxidative modifications, have been identified in aging and cataractous human lens proteins (Chen et al., 2017; Wilmarth et al., 2006; Yanshole et al., 2013). Acylation is another PTM that is prevalent at lysine residues of cellular proteins (James et al., 2018; Thinon and Hang, 2015). Modifications from acylation include acetylation, succinylation, propionylation, malonylation and glutarylation (Chen et al., 2007; Peng et al., 2011; Thinon and Hang, 2015). These acylation modifications occur in lysine residues and are mediated by enzymatic and nonenzymatic mechanisms with acetyl-CoA as the acyl donor.
We previously showed that acetyllysine (AcK) and succinyllysine (SuccK) are found in human lenses (Nagaraj et al., 2012; Nandi et al., 2019b). We identified AcK and SuccK at specific lysine residues in lens proteins by mass spectrometric techniques (DiMauro et al., 2014; Nagaraj et al., 2012; Nandi et al., 2019b). We found that lysine acetylation improves the chaperone activity of α-crystallin (Nagaraj et al., 2012; Nahomi et al., 2013a; Nandi et al., 2019a) and prevents glycation-mediated modification of lens proteins (Nahomi et al., 2013b). In addition, we demonstrated that acetylation of the mini-chaperone peptides from the core domain of α-crystallin inhibits apoptosis of lens epithelial cell better than unacetylated native peptides (Nahomi et al., 2013c). In another study, we showed that acetylation of K2 in γD-crystallin leads to compromised thermotolerance (DiMauro et al., 2014). We found that SuccK modifications are predominant in αB-crystallin and are present in very young lens proteins and persist throughout life (Nandi et al., 2019b). SuccK modification also improves the chaperone activity of αB-crystallin (Nandi et al., 2019b). In our recent work, mice lacking SIRT3 and SIRT5 had more AcK modifications in their lens proteins than did WT mice (Nandi et al., 2019a), suggesting that sirtuins could reverse acetylation modification.
Whether malonylation and propionylation occur in human lens proteins is not known. The acyl donors for lysine malonylation and propionylation are malonyl-CoA and propionyl-CoA, respectively, which are key metabolites in the Krebs cycle, amino acid degradation and fatty acid oxidation (Grevengoed et al., 2014; Hirschey and Zhao, 2015; Peng et al., 2011). These modifications have functional implications for proteins. For example, a recent study demonstrated that malonylation of GAPDH at K213 regulates its interaction with mRNA, promoting inflammation (Galvan-Pena et al., 2019). Malonylation K1218 of mTOR has been implicated in angiogenesis (Bruning et al., 2018). Further, lysine malonylation of histones has possible implications in diabetes and obesity (Nie et al., 2017). Kebede et al. demonstrated the role of histone propionylation in the transcription and regulation of chromatin structure and function (Kebede et al., 2017). Fritz et al. demonstrated that chronic ethanol ingestion induced propionylation in mouse liver mitochondria (Fritz et al., 2013).
The sirtuin family of HDACs comprises regulators of acyl modifications (Frye, 1999, 2000). They are NAD+-dependent enzymes that support cell survival and regulate metabolism (Hirschey, 2011). There are 7 sirtuins (SIRT1–7) with various subcellular localizations (Newman et al., 2012). Sirtuins are involved in removing the acyl groups from acyl-lysine moieties, thereby regulating acylation levels in proteins (Denu, 2005; Sauve et al., 2006). Lysine demalonylation is carried out by SIRT5 (Du et al., 2011). Four isoforms of SIRT5 have been identified in humans (Du et al., 2018) with molecular weights of 33.8, 32.6, 31.9 and 21.6 kDa, as reported in UniProtKB. In addition to demalonylase activity, SIRT5 is also known to exhibit deacetylase (Nakagawa et al., 2009) and desuccinylase activity (Du et al., 2011). Lysine depropionylation is mediated mainly by SIRT3 (Fritz et al., 2013; Garrity et al., 2007). In humans, thus far, 2 isoforms of SIRT3 have been identified and have molecular weights of 43.5 and 28.5 kDa (Scher et al., 2007). It controls a wide range of biological functions that include gene expression, neuroprotection and aging (Ansari et al., 2017; Kong et al., 2010; Shi et al., 2005). In addition to lysine depropionylation, SIRT3 also deacetylates proteins (Lombard et al., 2007).
In this study, we report that MalK and PropK are present in all major crystallins and that both SIRT3 and SIRT5 are present in human lens epithelial cells. Furthermore, the absence of SIRT3 and SIRT5 in mice leads to higher levels of MalK- and PropK-modified proteins in lenses.
2. Materials and methods
2.1. Materials
Malonyl-CoA, propionyl-CoA, NAD+ and a protease inhibitor cocktail mixture were obtained from MilliporeSigma (St. Louis, MO). Malonyllysine (Cat# PTM-901) and propionyllysine (Cat# PTM-201) antibodies were purchased from PTM Biolabs (Chicago, IL). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Cat# AB2302) was purchased from MilliporeSigma. All other antibodies were from Cell Signaling Technology (Danvers, MA): β-actin (Cat# 4970L), histone H3 (Cat# 4499S), superoxide dismutase 2 (SOD2, Cat# 1314S), SIRT3 and 5 (Antibody Sampler Kit Cat# 9787) and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Cat# 7074S). Recombinant His-tagged SIRT3 (Cat# 10011194) and GST-tagged SIRT5 (Cat# 10318) were from Cayman Chemical (Ann Arbor, MI). All other chemicals used in this study were of analytical grade.
2.2. Immunohistochemistry for MalK and PropK in human lenses
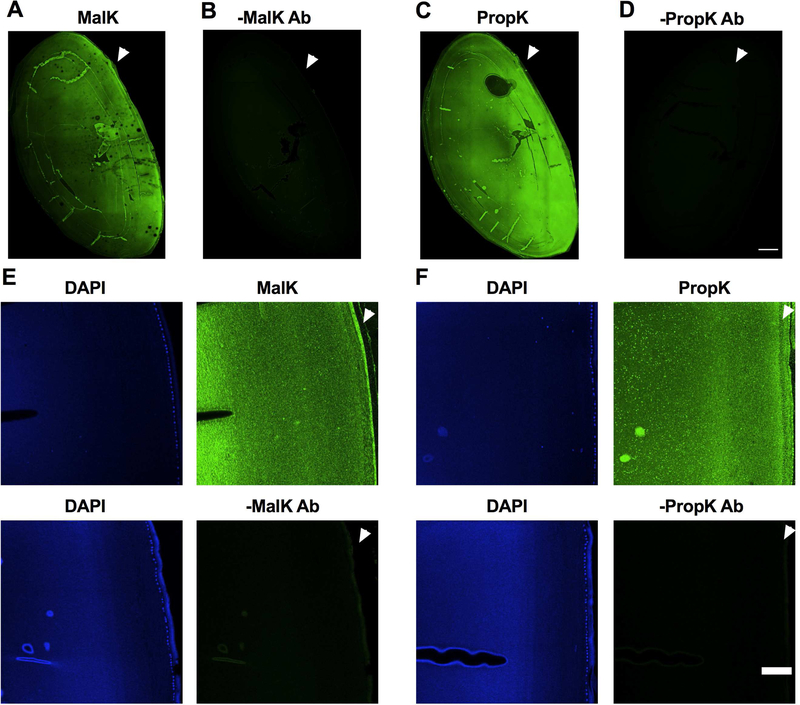
Paraffin-embedded human eye lenses were sectioned, deparaffinized and subjected to high-temperature antigen retrieval in 10 mM citrate buffer, pH 6.0. Sections were then blocked with 5% normal goat serum (Life Technologies, Carlsbad, CA) in PBS for 1 h. Sections were incubated for 48 h at 4°C with either the antibody for MalK or PropK (both diluted 1:50 in 5% normal goat serum), followed by incubation for 2 h at 37°C with Alexa Fluor 488 conjugated goat antirabbit IgG (1:250 dilution, Life Technologies, Cat#A11001). Nuclei were stained with DAPI/Vectashield. The images were recorded with an inverted Olympus FV1000 scanning confocal microscope using a 10X 0.4NA objective (Fig. 1 A to D) or 20X 0.75NA objective (all panels in Fig. 1 E and F). The tiled images were recorded with 10% overlap in x and y direction. The individual image tiles were background and flatfield corrected with the BaSiC plugin in Fiji and stitched with the Stitching tool in Fiji (Peng et al., 2017; Rueden et al., 2017; Schindelin et al., 2012). Sections stained with secondary antibody alone served as negative controls.
Fig. 1. Immunohistochemical detection of MalK and PropK modified proteins in the human lens.
Immunohistochemical analysis of a human lens transverse sections (donor age: 60 years) showed immunoreactivity (green) for MalK (A) and PropK (C) throughout the lens. Images of higher magnification showed immunoreactivity in both epithelial and cortical fiber cells (E and F top panels). Arrowhead points to the anterior region of the lens. Sections without the primary antibody (negative control) showed no immunoreactivity (B and D as well as bottom panels of E and F). DAPI staining (blue) was used to show the nuclei of epithelial cells. Scale bar = 500 μm for A to D and 100 μm for the images in panels of E and F.
2.3. Isolation of water-soluble (WS) and water-insoluble solubilized (WIS) proteins from human lenses and WS proteins from mouse lenses
Human lenses were obtained from Saving Sight, Kansas City, MO, and Rocky Mountain Lions Eye Bank, Aurora, CO. Lenses were stored at −80°C until use. Water-soluble (WS) proteins and water-insoluble proteins solubilized by sonication (WIS) were prepared from decapsulated lenses as previously described (Nagaraj et al., 2012). Mouse lenses were from 2- to 3-month-old wild-type (WT) C57BL/6J strain (Jackson Laboratories, Bar Harbor, ME), SIRT3 knockout (KO) and SIRT5 KO mice (C57BL/6J strain, were kind gifts from Dr. Fred Alt, Boston Children’s Hospital, and Dr. Eric Verdin, The Buck Institute for Research on Aging). WS proteins were isolated from these lenses by homogenization in 50 mM Tris-HCl buffer, pH 8, containing 1.5 mM KCl, 1 mM ΜgCl2, and 0.5 mM DTT, as previously described (Nandi et al., 2019a).
2.4. Isolation of cytosolic, mitochondrial and nuclear fractions from human lens epithelial cells
Human lens epithelial cells (FHL124 from Dr. Michael Wormstone, University of East Anglia, UK, originally from Prof. John Reddan, Oakland University, MI) were cultured in 5% FBS minimal essential medium with gentamicin/L-glutamate (1:100) in 100 mm tissue culture dishes. Subcellular fractions were prepared using a ProteoExtract subcellular protein extraction kit (MilliporeSigma, Cat# 539790) according to the manufacturer’s instructions. The purity of the fractions was checked by western blot analysis as described below using the following antibodies: cytosolic GAPDH (diluted 1:5,000), mitochondrial SOD2 (diluted 1:2,000) and nuclear histone H3 (diluted 1:2,500). Total cell lysate was prepared using 1X RIPA buffer (Thermo Scientific, Waltham, MA, Cat# 89900) containing a protease inhibitor cocktail mixture (1:100).
2.5. Malonylation and propionylation of lysozyme
Lysozyme from egg white (MilliporeSigma, 1 μg/μl) was incubated with 0.01 mM malonyl-CoA or 0.1 mM propionyl-CoA for 4 h at room temperature in PBS and dialyzed overnight against PBS at 4°C.
2.6. Western blotting
We used seven lenses (donor age: 17, 26, 37, 43, 55, 67 and 71 years) to determine MalK or PropK in WS and WIS. To determine the presence of SIRT3 and SIRT5 in WS, we used six lenses from donors (age: 9, 18, 30, 46, 62 and 73 years). None of these lenses were from diabetic donors. Proteins (10 to 50 μg) were subjected to SDS-PAGE (12% gels) and electrophoretically transferred to nitrocellulose membranes. The membranes were blocked with 5% blocking-grade nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) and incubated with one of the following primary antibodies overnight at 4°C: MalK (diluted 1:2,500), PropK (diluted 1:5,000), SIRT3 (diluted 1:1,000) and SIRT5 (diluted 1:1,000). Membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (diluted 1:5,000) for 1 h at room temperature. The immunoreactivity was detected using SuperSignal West Pico or Femto Kit (Pierce Chemicals, Rockford, IL). Membranes were stained with Ponceau S stain to show protein loading.
2.7. Specificity of immunoreaction for MalK and PropK
The lysozyme was acylated as described above. One hundred micrograms of malonylated lysozyme was incubated with 1 μg of MalK antibody or 50 μg of propionylated lysozyme was incubated with 1 μg PropK antibody in 200 μl PBS in a shaker for 24 h at 4°C. Western blotting was performed as described above using either 25 μg WS proteins (donor age: 37, 43 and 55 years) or 15 μg protein from the human lens epithelial cell lysate. Five micrograms of malonylated lysozyme and 0.5 μg of propionylated lysozyme were also processed similarly. After electrophoretic transfer to nitrocellulose membranes and blocking with 5% blocking grade nonfat dry milk, the membranes were cut into two halves. One part of each membrane was incubated with MalK antibody (diluted 1:5,000), and the other part was incubated with MalK antibody preincubated with malonylated lysozyme. Similarly, we incubated membranes with PropK (diluted 1:5,000) or PropK antibody preincubated with propionylated lysozyme. After secondary antibody treatment, membranes were developed as described above. In some cases, membranes were stripped and reprobed using β-actin antibody (1:5,000) and developed as described above.
2.8. Extraction of proteins from human epithelial cells, human lens and mouse heart for sirtuin activity assays
Experiments involving animals were approved by the Institutional Animal Care and Use Committee of the University of Colorado and were performed in accordance with the guidelines of NIH and ARVO. The epithelial cell lysate was prepared in 10 mM HEPES buffer, pH 8, containing 1. 5 mM KCl, 1 mM ΜgCl2, and 0.5 mM DTT by sonication followed by centrifugation at 20,000 x g for 10 min at 4°C as previously described (Nandi et al., 2019a). WS proteins from human lenses (donor age: 22, 39 and 71 years) and mouse heart tissue from C57BL/6J mice (2 or 3 months old) were prepared as previously described (Nandi et al., 2019a; Nandi et al., 2019b). The depropionylase and demalonylase activity were measured against propionylated and malonylated lysozyme, respectively. Propionylation or malonylation of the lysozyme was performed as described above, except for an overnight dialysis step at 4°C in buffer A. To assess the depropionylase and demalonylase activity of sirtuins in the epithelial cell lysate and WS human lens proteins, 50 μl of reaction mixture containing the WS proteins from lenses (1,000 μg), epithelial cell lysate (300 μg) or WS mouse heart tissue (positive control, 300 μg) with NAD+ (1 mM) and propionylated or malonylated lysozyme (5 μg) were incubated for 14 h at 37°C in buffer A. Depropionylation or demalonylation of the respective lysozyme was assayed by western blotting using a PropK or MalK antibody.
2.9. Synthesis of Nε-Malonyl-l-Lysine (MalK) and Nε-Propionyl-l-Lysine (PropK)
Syntheses of MalK and PropK were carried out according to a modified literature protocol (synthesis of ε-N-propionyl-, ε-N-butyryl-, and ε-N-crotonyl-lysine containing histone H3 using the pyrrolysine system (Gattner et al., 2013). The details of the protocols are described below.
2.10. Synthesis of Nε-Malonyl-l-Lysine (MalK)
Isolation of Nε-malonyl-Nα-boc-l-lysine —
246 mg (1 mmole) Nα-boc-l-lysine was dissolved in 5 μl 1 M sodium hydroxide and 5 μl THF and stirred on ice. To the mixture, 2 mmole of malonyl chloride in 1 ml THF was added. After the addition, the reaction mixture was stirred for another hour on ice before maintaining for 24 h at room temperature. The reaction mixture was diluted with 25 ml water and neutralized with HCl. The THF was removed under reduced pressure, and the remaining solution was lyophilized. The crude product was dissolved in 4 ml water and fractionated by preparative HPLC. Preparative HPLC runs were carried out with a binary pump (Waters 1525) operating at a flow rate of 15 ml/min. The sample was applied via a 2-μl injection loop (Rheodyne). Separations were carried out at room temperature on an RP C18 column (XBridge Prep C18, 250 × 19 mm, 5 (μm; Waters) connected to a guard column. After the initial column run, 0.3 ml/min was diverted through a valve to a UV-visible detector (Waters 2489), and the rest of the flow (14.7 ml/min) was collected in fractions. The following conditions were used for preparative purification of Nε-malonyl-l-lysine. Water (solvent A) and 80% acetonitrile (solvent B, v/v) were used as eluents. To both solvents, 0.1% formic acid (v/v) was added. The gradient program was: 2% B (0–5 min) to 10% B (20 min) to 30% B (30 min) to 60% B (40 min) to 100% B (45–55 min). The detection wavelength was set to 210 nm. Fractions were checked by LC-MS, and fractions showing a peak with an m/z of 333 and 355 (sodium adduct) were pooled. The methanol was removed under reduced pressure. The lyophilization yielded a brown oil (28 mg, 0.08 mmole, 8% yield). 1H NMR (500 MHz, D2O): 1.44 (s, 9H), 1.46 (m, 2H), 1.57 (m, 2H), 1.71 (m, 1H), 1.84 (m, 1H), 3.24 (t, J=7.1 Hz, 2H), 3.37 (s, 2H), and 4.07 (t, J=7.2 Hz, 1H). 13C NMR (125 MHz, D2O): 24.9, 30.2, 30.3, 32.9, 41.8, 56.4, 56.6, 84.0, 160.4, 171.5, 174.8, and 179.8. Fourteen mg (0.04 mmole) Nε-malonyl-Nα-boc-l-lysine was deprotected with 500 μl 3 M HCl for 30 min at room temperature. Then, the solution was neutralized and fractionated by flash chromatography (RP C18, 5% acetonitrile + 0.1% trifluoroacetic acid (v:v)). Fractions were analyzed by TLC (RP C18, 5% acetonitrile + 0.1% trifluoroacetic acid (v:v), ninhydrin staining), and spot fractions with Rf=0.9 were pooled and lyophilized. Nε-Malonyl-l-lysine was obtained as a yellowish solid (3 mg, 0.012 mmoles, 31% yield). 1H NMR (500 MHz, D2O): 1.62 (m, 2H), 1.77 (m, 2H), 2.10 (m, 2H), 3.42 (t, J=7.5 Hz, 2H), 3.58 (s, 2H), 4.09 (t, J=7.3 Hz, 1H). 13C NMR (125 MHz, D2O):24.5, 30.5, 32.5, 41.8, 56.7, 56.8, 171.6, 175.0, 176.5.
2.11. Synthesis of Nε-Propionyl-l-Lysine (PropK)
Isolation of Nε-Propionyl-Nα-boc-l-Lysine —
246 mg (1 mmole) Nα-boc-l-lysine was dissolved in 5 ml 1 M NaOH and 5 ml THF and stirred on ice. Then, 100 μl (1 mmole) of propionyl chloride was added stepwise in 10 μl aliquots. The reaction mixture was then stirred for 24 h at room temperature. The solution was neutralized, and THF was removed under reduced pressure. The remaining solution was lyophilized. The crude product was suspended in 2 ml water/acetonitrile (1:1, v:v) and fractionated by flash chromatography (silica gel, ethyl acetate + 1% formic acid (v:v)). Fractions were analyzed by TLC (silica gel, ethyl acetate + 1% formic acid (v:v), ninhydrin staining), and spot fractions with Rf=0.4 were pooled. Evaporation of the solvent yielded a white powder (220 mg, 0.73 mmole), 73% yield). 1H NMR (500 MHz, D2O): 1.11 (t, J=7.3 Hz, 3H),1.40 (m, 2H), 1.44 (s, 9H), 1.54 (m, 2H), 1.71 (m, 1H), 1.83 (m, 1H), 2.23 (q, J=7.8 Hz, 2H), 3.19 (t, J=6.5 Hz, 2H), and 4.08 (t, J=7.3 Hz, 1H). 13C NMR (125 MHz, D2O): 12.9, 24.9, 29.7, 30.7, 31.8, 41.5, 42.6, 55.7, 84.0, 160.4, 179.4, and 180.4. Sixty mg (0.20 mmole) Nε-propionyl-Nα-boc-l-lysine was deprotected with 500 μl 3 M HCl for 30 min at room temperature. Afterwards, the solution was neutralized and fractionated by flash chromatography (RP C18, 5% acetonitrile + 0.1% trifluoroacetic acid (v:v)). Fractions were checked by TLC (RP C18, 5% acetonitrile + 0.1% trifluoroacetic acid (v:v), ninhydrin staining), and spot fractions with Rf=0.9 were pooled and lyophilized. Nε-Propionyl-l-lysine was obtained as a white powder (38 mg, 0.19 mmol, 95% yield). 1H NMR (500 MHz, D2O): 1.10 (t, J=7.1 Hz, 3H), 1.44 (m, 2H), 1.57 (m, 2H), 1.93 (m, 1H), 1.98 (m, 1H), 2.66 (q, J=7.2 Hz, 3H), 3.19 (t, J=7.5 Hz, 2H), and 4.04 (t, J=7.4 Hz, 1H). 13C NMR (125 MHz, D2O): 12.8, 24.1, 32.0, 33.0, 41.3, 42.6, 56.2, 174.9, and 180.6.
2.12. LC-MS/MS Quantification of MalK and PropK in Human Lenses
Hydrolysis of Human Lens Proteins —
We have used a total of 24 decapsulated lenses (donor age: 20, 25, 29, 32, 37, 38, 43, 49, 51, 52, 55, 58, 58, 60, 63, 66, 66, 67, 69, 70, 70, 71 73 and 75 years) were weighed and homogenized in 1 ml argon-saturated PBS containing 1 mM EDTA. Three hundred microliters of the homogenate was dialyzed against 20 mM phosphate buffer, pH 7.4, and lyophilized. Enzymatic digestion of lens proteins was performed as described previously with some minor modifications (Smuda et al., 2015). Five hundred micrograms of freeze-dried protein was dissolved in 150 μl PBS, and one small crystal of thymol was added. To this solution, 0.1 unit of pronase E (two additions), 0.3 unit of leucine aminopeptidase, and 0.3 unit of carboxypeptidase Y were added stepwise at 24 h intervals for 96 h. Finally, the sample was filtered through a 3 kDa molecular weight cut-off filter (VWR, Radnor, PA). To determine protein digestion efficiency, we compared the Nε-carboxymethyllysine (CΜL) levels in the enzyme digests with those in the acid hydrolysates of the same sample and considered CΜL levels in acid-hydrolyzed samples to represent 100% hydrolysis efficiency, as described previously (Smuda et al., 2015). Briefly, 500 μg protein powder was hydrolyzed with 6 M HCl at 110°C for 24 h. The samples were dried in a Speed Vac to remove the HCl and then dissolved in water.
LC-MS/MS Analyses —
Sample aliquots were diluted with water prior to LC-MS/MS analysis. Chromatography analyses were carried out on a Waters ACQUITY UPLC system (Milford, MA) connected to a Sciex 4500 QTrap mass spectrometer (Redwood City, CA). Chromatographic separations were carried out on an ACQUITY HSS T3 column (100 × 2.1 mm, 1.8 μm, Waters, Milford, MA) connected to a guard column using a flow rate of 0.6 ml/min. Water (solvent A) and 80% acetonitrile in water (solvent B, v/v) were used as eluents. To both solvents, 0.12% heptafluorobutyric acid (v/v) was added. Analyses were performed at a column temperature of 40°C using the following elution gradient: 2% B (0 to 2.2 min) to 8% B (3.3 min) to 34% B (7.6 min) to 100% B (7.8 to 9.5 min). The column was equilibrated with 2% B for 2.5 min prior to initiating the next analysis. Detection of MalK (tR=3.2 min), PropK (tR=5.0 min) and CΜL (tR=2.3 min) was achieved using multiple reaction monitoring. The ion source was run through the column under the following conditions: temperature, 650°C; ion spray voltage, 2.5 kV; curtain gas, 35 ml/min; nebulizer gas, 65 ml/min; and heating gas, 70 ml/min. The declustering potential for MalK was set to 30 V, for PropK to 22 V, and for CΜL to 40 V. The MRM parameters were as follows: Q1→Q3 [m/z], collision energy [eV], and cell exit potential [V]. MalK: quantifier, 232.1→84.0, 32, 12; qualifier 1, 233.1→126.0, 18, 12; and qualifier 2, 233.1→170.0, 20, 15. PropK: quantifier, 203.0→84.1, 27, 10; qualifier 1, 203.0→140.1, 20, 12; and qualifier 2, 203.0→157.0, 14, 13. CΜL: quantifier, 205.1→130.2, 17, 11; qualifier 1, 205.1→84.1, 25, 13; and qualifier 2, 205.1→56.1, 50, 10. Quantitation was performed based on the standard addition method.
2.13. Mass spectrometric identification of MalK and PropK sites in human lens proteins
Human lens WS proteins (5 mg) were from 26- and 71-year-old donors. WS proteins were digested using the urea digest protocol provided with the kit from Cell Signaling Technologies. Briefly, the WS protein was solubilized and denatured using a urea lysis buffer with protease inhibitors before being reduced and alkylated with dithiothreitol (DTT) and iodoacetamide. Samples were diluted to a final concentration of 2 M urea with 20 mM HEPES buffer before being digested overnight at room temperature with 1:40 trypsin-to-substrate on a platform rocker set to 30 RPM. The resulting peptide solutions were desalted using a 0.7 ml C18 Sep-Pak column (Waters Corporation, Milford, MA), and the eluted peptides were lyophilized.
Immunoprecipitation for peptides bearing MalK or PropK modifications was performed using both PTMScan malonyl-lysine [Mal-K] (Cat# 93872) and PTMScan® propionyl-lysine [Prop-K] (Cat #17848) kits from Cell Signaling Technology according to the manufacturer’s protocol. Briefly, the beads were washed with PBS four times before incubation with the sample for 2 h at 4°C on a shaker. After incubation, the supernatant was removed, and the beads were washed twice with ice-cold 1X IAP buffer and then three times with ice cold water (Honeywell Inc. Burdick and Jackson, Morris Plains, NJ). The peptides were eluted by two washes with 0.15% TFA, pooled, and purified with a Pierce C18 spin column (Thermo Fisher Scientific Inc.) before being evaporated to dryness in a Speed Vac concentrator at 25°C. The peptides were resuspended in 3% acetonitrile in 0.1% formic acid for MS analysis.
The enriched MalK and PropK peptide samples were loaded onto a 2 cm PepMap 100 nanoViper trapping column and chromatographically resolved on-line using a 0.075 × 250 mm, 2.0 μ Acclaim PepMap RSLC reversed-phase nano column (Thermo Scientific) using a 1290 Infinity II LC system equipped with a nanoadapter (Agilent). Mobile phases consisted of water + 0.1% formic acid (A) and 90% aq. acetonitrile + 0.1% formic acid (B). Columns were heated to 50°C. Samples were loaded onto the trapping column at 3.2 μl/min for 3.2 min under the initial conditions before being chromatographically separated at an effective flow rate of 330 nl/min using a the following gradient: 3–8% B for 1 min, 8–28% B over 26 min, and 28–40% B over 3 min for a total 30 min. The gradient method was followed by a column wash with 75% B for 5 min. Data were collected on a 6550 Q-TOF equipped with a nano source (Agilent) operated using intensity-dependent CID MS/MS to generate peptide IDs. The capillary voltage, drying gas flow, and drying gas temperature were set to 1300 V, 11.0 l/min and 200°C, respectively. MS/MS data were collected by positive ion polarity over mass ranges of 260–1700 m/z at a scan rate of 10 spectra/sec for the MS scans and mass ranges of 50–1700 m/z at a scan rate of 3 spectra/sec for the MS/MS scans. All charge states were allowed, except that singly charged species were excluded from being selected during MS/MS acquisition, and charge states 2 and 3 were given preference. SpectrumMill software (Agilent) was used to extract, search, and summarize peptide identity results. Spectra were compared against the SwissProt Homo sapiens database, allowing up to 4 missed tryptic cleavages with fixed carbamidomethyl (C) and variable deamidated (NQ), oxidation (M), and either malonyl (K) or propionyl (K) modifications, depending on the sample. The allowed monoisotopic peptide mass tolerance was ± 20.0 ppm, and the MS/MS tolerance was ± 50 ppm. A minimum peptide score of 8, scored peak intensity of 50%, FDR less than 1.2% and a variable modification localization (VΜL) score greater than 1.15 were used as cut-offs for determining quality PTM peptide hits. Scored peak intensity is a measure of how much of the extracted MS/MS spectrum from the sample run matches the database match or theoretical spectrum. The VΜL score is an automated feature that assigns modifications, e.g. MalK or PropK, to a specific lysine residue in a peptide sequence when there are two or more possible lysine position assignments. A score greater than 1.15 indicates, with confidence, that there is at least one distinguishing y or b ion in the extracted spectrum that allows the PTM to be mapped to a specific lysine (See tables 1–4). For MalK or PropK peptides reported by the database search engine to be unique to either aged or young samples, precursor ion chromatogram peaks were manually extracted from the raw data from both sample types to verify uniqueness. If an extracted ion chromatogram (EIC) was found in both samples at the correct retention time, either the precursor was not selected by the MS/MS acquisition algorithm or the spectral quality was too low for a high confidence identification. Such modified peptides are likely not differential between sample types and were marked as “EIC” in the table. If the presence/absence of an EIC was in agreement with the database search results, the modified peptide is marked as “Flagged”.
Table 1.
MalK and PropK modification sites in α-crystallins.
| Malonylation | Propionylation | ||||
|---|---|---|---|---|---|
| Protein | Mod Site | Age 26 | Age 71 | Age 26 | Age 71 |
| αA-crystallin | K70 | - | - | Flagged | ms/ms |
| K99 | - | - | ms/ms | ms/ms | |
| K166 | ms/ms | ms/ms | ms/ms | EIC | |
| αB-crystallin | K72 | - | - | Flagged | ms/ms |
| K90 | ms/ms | ms/ms | ms/ms | ms/ms | |
| K92 | ms/ms | ms/ms | EIC | ms/ms | |
| K103 | - | - | ms/ms | ms/ms | |
| K166 | ms/ms | ms/ms | ms/ms | ms/ms | |
| K174 | - | - | EIC | ms/ms | |
Mod Site text Black – Lysine site found to be both malonylated and propionylated
Mod Site text Blue – Lysine site found to be only malonylated
Mod Site text Red – Lysine site found to be only propionylated
ms/ms – quality fragmentation spectrum match that passed quality Peptide Score, SPI%, and VΜL score cut-off filters
EIC – Extracted Ion Chromatogram (EIC) peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
Flagged –No EIC peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
Table 4.
PropK modification sites in cytoskeletal proteins.
| Malonylation | Propionylation | ||||
|---|---|---|---|---|---|
| Protein | Mod Site | Age 26 | Age 71 | Age 26 | Age 71 |
| Filensin | K287 | - | - | Flagged | ms/ms |
| Phakinin | K143 | - | - | Flagged | ms/ms |
Mod Site text Black – Lysine site found to be both malonylated and propionylated
Mod Site text Blue – Lysine site found to be only malonylated
Mod Site text Red – Lysine site found to be only propionylated
ms/ms – quality fragmentation spectrum match that passed quality Peptide Score, SPI%, and VΜL score cut-off filters
EIC – Extracted Ion Chromatogram (EIC) peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
Flagged –No EIC peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
2.14. Statistical analysis
The data are presented as the means ± SD from the number of experimental replicates indicated in the figure legends. Statistically significant differences between the groups were analyzed by Student’s t-test using GraphPad Prism 8 software. A p-value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Immunohistochemical detection of MalK and PropK in human lenses.
To determine the morphological distribution of MalK and PropK modified proteins, we used transverse sections from a 60-year-old lens. As shown in Fig. 1A and C, we found immunoreactivity for both MalK and PropK (green) throughout the lens and the zoomed images showed immunoreactivity in both epithelial and fiber cells (Fig. 1E and F top panels). Such immunoreactivity was absent in sections treated with secondary antibody alone (Fig. 1B and D, and Fig. 1E and F bottom panels).
3.2. Effect of age on MalK and PropK modification in human lens proteins.
First, we examined the cross-reactivity of antibodies using the MalK- or PropK-modified lysozyme. Our results showed no reactivity with the MalK antibody against the propionylated lysozyme; similarly, no reactivity was observed for the PropK antibody against malonylated lysozyme (Fig. S1). These results suggested that MalK and PropK antibodies are specific to those modifications.
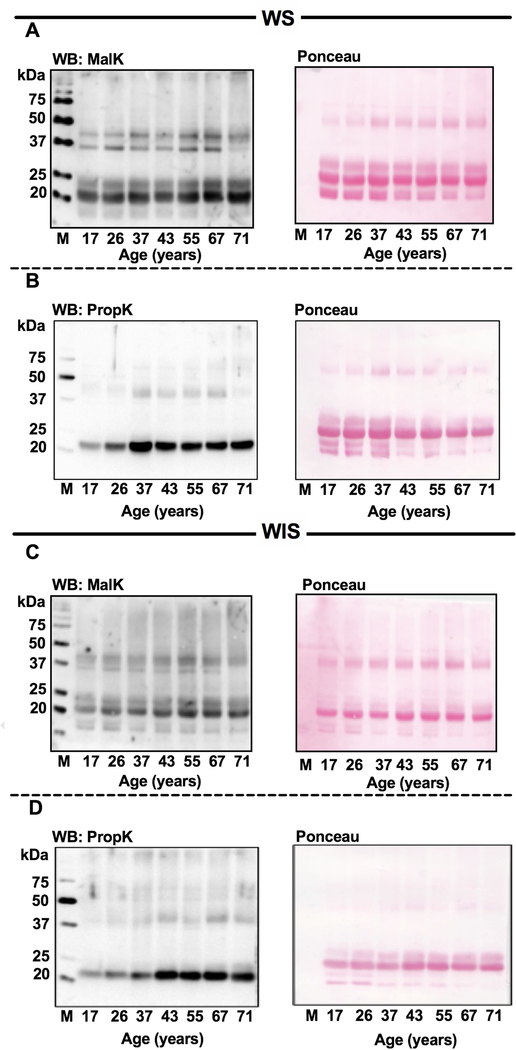
Western blotting data showed MalK and PropK-modified proteins in WS (Fig. 2A and B) and WIS (Fig. 2C and D). In both WS and WIS, crosslinked proteins showed higher MalK modification than PropK modification. No apparent changes in either MalK or PropK levels during aging were observed. Densitometric analyses (normalized to the protein in the Ponceau-stained membranes) revealed that the MalK and PropK immunoreactivity in the WS proteins was reduced by 84% and 97%, respectively, by prior incubation of the antibody with malonylated or propionylated lysozyme (Fig. S2, A and B). As expected, prior incubation of the antibody with malonylated or propionylated lysozyme reduced immunoreactivity against the malonylated or propionylated lysozyme by 65% and 99%, respectively. Together, these results suggested that the immunoreactivity observed in human lens proteins (Fig. 2) is largely due to the MalK- and PropK-modified proteins and not due to nonspecific reactions of the antibodies.
Fig. 2. Western blots for MalK and PropK-modified proteins in the water-soluble (WS) and water insoluble-solubilized (WIS) fractions.
Human lens proteins were subjected to western blotting for MalK (A, WS and C, WIS) and PropK (B, WS and D, WIS). Ponceau stained membranes (right) to show protein loading. M, molecular weight markers.
3.3. LC-MS/MS quantification of MalK and PropK in human lens proteins.
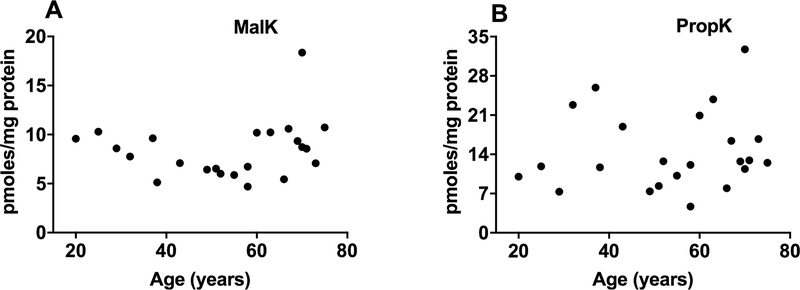
For these analyses, we used enzyme-digested lens homogenates (not separated into WS and WIS protein fractions). We found both modifications by LC-MS/MS (Fig. 3A and B). The PropK levels (4.7–32.7 pmoles/mg lens protein) were slightly higher than the MalK levels (4.6–18.4 pmoles/mg lens protein).
Fig. 3. LC-MS/MS quantification of MalK and PropK in aging human lens proteins.
The levels of MalK (A) and PropK (B) in enzyme digested human lens proteins are shown.
3.4. Identification of MalK and PropK residues in human lens by Q-TOF tandem MS/MS mass spectrometry.
To identify MalK and PropK in lens proteins, we used WS proteins from a 26- and a 71-year-old lens. MalK was detected at K166 of the αΑ-crystallin and at K90, K92 and K166 of the αB-crystallin of both lenses (Table 1). PropK was detected at K99 and K166 of αΑ-crystallin in both lenses. PropK at K70 was only found in the 71-year old lens. In αB-crystallin, we detected one more PropK site in the 71-year old lens at K72, whereas K90, K92, K103, K166 and K174 were found in both 26- and 71-year old lenses.
We also detected MalK and PropK modified sites in β- and γ-crystallins. We detected MalK modified sites in βA3-crystallin at sites K44 and K125; in βB2-crystallin at K108, K120, K121 and K172; in γ-crystallin S at K7, K14, K95 and K159 (Table 2). Site K155 was found only in the 26-year old lens in γS-crystallin. Although there were not many changes in MalK site identification, we detected more PropK modified sites in βA3, βA4 and βB1-crystallin of the 71-year old lens than of the 26-year old lens. While we detected PropK at K118, K143, K187, K235 and K252 in βB1-crystallin of the 71-year old lenses, only sites K118 and K143 showed PropK modification in the 26-year old lens. PropK in βB2-crystallin was observed at 6 lysine residues (K76, K101, K120, K121, K168 and K172) in both young and old lenses. There were no differential site identifications in γ-crystallins. Site K163 in γC-crystallin and sites K14, K95, K154, K155 and K159 in γS-crystallin were found in both 26 and 71-year old lenses.
Table 2.
MalK and PropK modification sites in β and γ-crystallins
| Malonylation | Propionylation | ||||
|---|---|---|---|---|---|
| Protein | Mod Site | Age 26 | Age 71 | Age 26 | Age 71 |
| βA3-Crystallin | K44 | ms/ms | ms/ms | ms/ms | ms/ms |
| K122 | - | - | ms/ms | ms/ms | |
| K125 | ms/ms | EIC | Flagged | ms/ms | |
| K131 | - | - | ms/ms | ms/ms | |
| K193 | - | - | ms/ms | ms/ms | |
| βA4-Crystallin | K13 | - | - | Flagged | ms/ms |
| K118 | - | - | ms/ms | ms/ms | |
| βB1-Crystallin | K118 | - | - | ms/ms | ms/ms |
| K143 | - | - | EIC | ms/ms | |
| K187 | - | - | Flagged | ms/ms | |
| K235 | - | - | Flagged | ms/ms | |
| K252 | - | - | Flagged | ms/ms | |
| βB2-Crystallin | K76 | - | - | ms/ms | ms/ms |
| K101 | - | - | ms/ms | ms/ms | |
| K108 | ms/ms | EIC | - | - | |
| K120 | ms/ms | ms/ms | ms/ms | ms/ms | |
| K121 | ms/ms | ms/ms | ms/ms | ms/ms | |
| K168 | - | - | ms/ms | ms/ms | |
| K172 | ms/ms | ms/ms | ms/ms | ms/ms | |
| γC-Crystallin | K163 | - | - | ms/ms | EIC |
| γS-Crystallin | K7 | EIC | ms/ms | - | - |
| K14 | ms/ms | ms/ms | ms/ms | ms/ms | |
| K95 | ms/ms | EIC | ms/ms | ms/ms | |
| K154 | - | - | ms/ms | ms/ms | |
| K155 | ms/ms | flagged | ms/ms | ms/ms | |
| K159 | ms/ms | ms/ms | ms/ms | ms/ms | |
Mod Site text Black – Lysine site found to be both malonylated and propionylated
Mod Site text Blue – Lysine site found to be only malonylated
Mod Site text Red – Lysine site found to be only propionylated
ms/ms – quality fragmentation spectrum match that passed quality Peptide Score, SPI%, and VΜL score cut-off filters
EIC – Extracted Ion Chromatogram (EIC) peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
Flagged –No EIC peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
Several other cytosolic proteins also showed MalK and PropK modifications in both lenses (Table 3). Among the proteins, fructose-bisphosphate aldolase A and glyceraldehyde-3-phosphate dehydrogenase each had more than one MalK and PropK modified sites identified. In addition, two cytoskeletal proteins, filensin and phakinin, showed MalK- and PropK-modified sites in only the 71-year-old lens but not in the 26-year-old lens (Table 4).
Table 3.
MalK and PropK modification sites in other cytosolic proteins.
| Malonylation | Propionylation | ||||
|---|---|---|---|---|---|
| Protein | Mod Site | Age 26 | Age 71 | Age 26 | Age 71 |
| Fructose-bisphosate aldolase A | K42 | - | - | ms/ms | ms/ms |
| K108 | ms/ms | EIC | - | - | |
| K111 | ms/ms | flagged | ms/ms | EIC | |
| Fructose-bisphosate aldolase C | K111 | ms/ms | flagged | ms/ms | ms/ms |
| glutathione Synthetase | K186 | ms/ms | ms/ms | ms/ms | ms/ms |
| K443 | - | - | flagged | ms/ms | |
| Glyceraldehyde-3-phosphate dehydrogenase | K194 | - | - | ms/ms | ms/ms |
| K215 | ms/ms | ms/ms | ms/ms | ms/ms | |
| K219 | ms/ms | ms/ms | - | - | |
| Phosphoglycerate kinase 1 | K30 | ms/ms | EIC | EIC | ms/ms |
| K131 | ms/ms | EIC | - | - | |
| Phosphoglycerate mutase 1 | K100 | ms/ms | ms/ms | EIC | ms/ms |
| Quinone oxidoreductase PIG3 | K194 | ms/ms | EIC | ms/ms | EIC |
| Sorbitol dehydrogenase | K339 | - | - | ms/ms | ms/ms |
Mod Site text Black – Lysine site found to be both malonylated and propionylated
Mod Site text Blue – Lysine site found to be only malonylated
Mod Site text Red – Lysine site found to be only propionylated
ms/ms – quality fragmentation spectrum match that passed quality Peptide Score, SPI%, and VΜL score cut-off filters
EIC – Extracted Ion Chromatogram (EIC) peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
Flagged –No EIC peak present based on the retention time (less than 0.15 min shift) and mass (less than 5ppm) of the peptide matched during database searching in the other aged lens sample
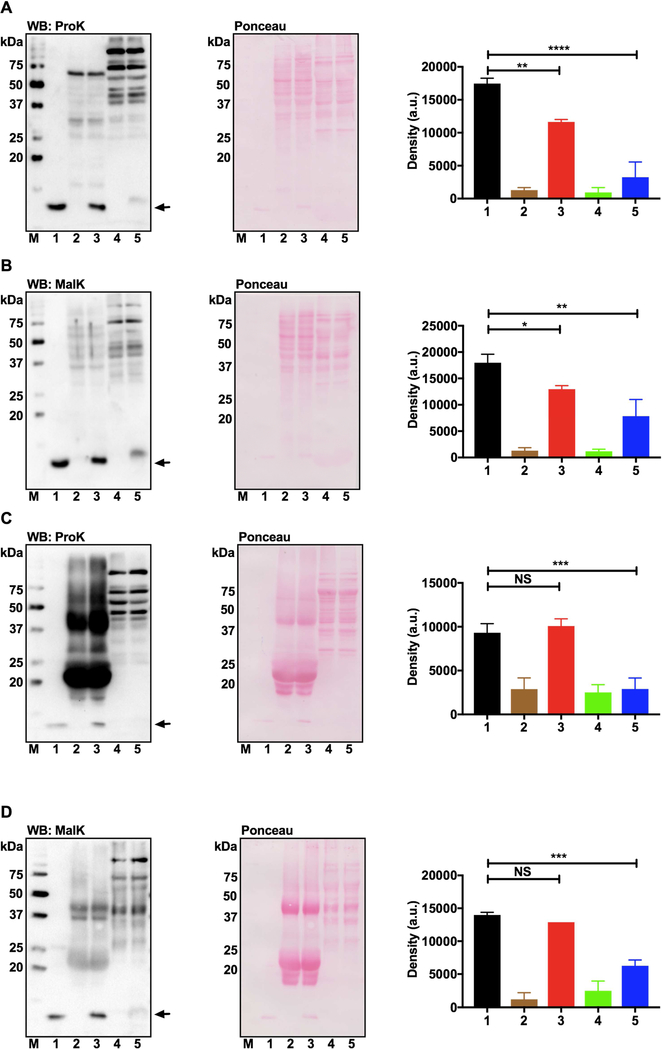
3.5. Proteins of human lens epithelial cells are propionylated and malonylated.
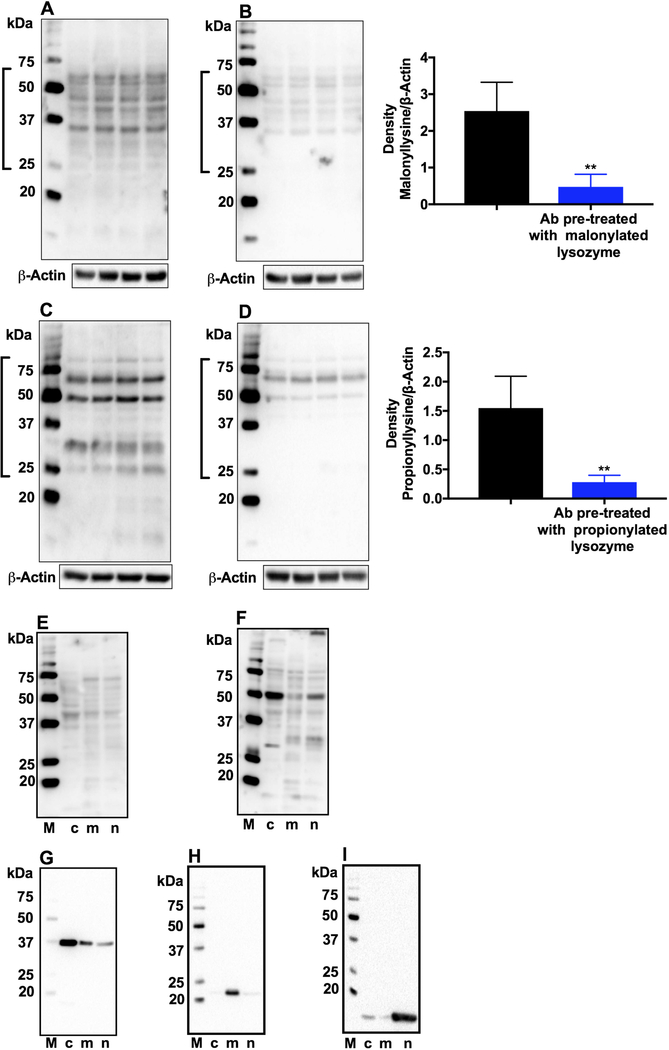
Western blot results of epithelial cell lysates showed several proteins to be malonylated and propionylated (Fig. 4A and C). The proteins did not appear to be crystallins, as there was hardly any immunoreactivity around the 20-kDa region. This immunoreactivity was specific, as demonstrated by immunoreactivity that was considerably reduced when MalK and PropK antibodies were preincubated with either malonylated or propionylated lysozyme before used for western blotting (Fig. 4B and D, and densitometric plots shown in the right panels).
Fig. 4. MalK- and PropK-modified proteins in human lens epithelial cells.
FHL124 cell lysates were subjected to western blotting. Membranes were incubated with the primary antibody for MalK or PropK (A and C) or with the primary antibody pre-incubated with malonylated or propionylated lysozyme (B and D). Densitometric plots for proteins within open brackets, normalized to β-actin (loading control) are shown on the right. **p<0.005. n=4. Subcellular fractions of FHL124 cells were analyzed for MalK (E) or PropK (F)-modified proteins by western blotting. To confirm fractionation, the cytosolic, mitochondrial and nuclear fractions were probed in western blots for GAPDH (G), SOD2 (H) and histone H3 (I), respectively. c, cytosolic; m, mitochondrial; n, nuclear. M, molecular weight markers.
Next, we investigated MalK- and PropK-modified proteins in the subcellular fractions of lens epithelial cells. We found MalK and PropK in the cytosolic, mitochondrial and nuclear fractions (Fig. 4E and F). Fractionation efficiency was confirmed by western blot analysis, which showed GAPDH primarily in the cytosol and, to a lesser extent, in the mitochondria and nucleus (Fig. 4G). Similarly, SOD2 and histone H3 were predominantly present in the mitochondria and nucleus, respectively (Fig. 4H and I).
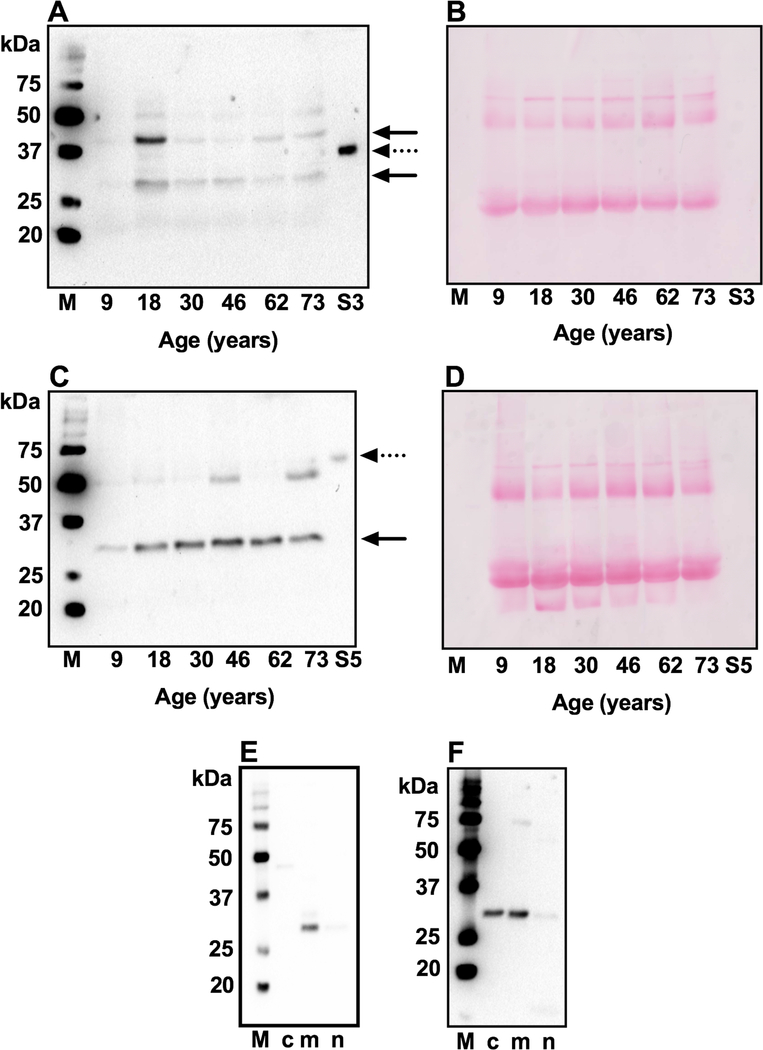
3.6. Sirtuins are present in human lens and epithelial cells.
Western blot analysis of WS protein fractions from human lenses showed immunoreactivity against SIRT3 for two proteins: at ~31 and ~43 kDa (Fig. 5A) and against SIRT5 for a protein at ~30 kDa (Fig. 5C). The two bands observed for SIRT3 could be due to two splice variants of the protein. An additional protein at ~50 kDa also indicated an immunoreaction against SIRT5 in some lenses. There were no apparent age-related differences in the levels of the two proteins. Membranes were stained with Ponceau S to show equal protein loading (Fig. 5B and D). We further determined the locations of the two sirtuins in cytosolic, mitochondrial and nuclear fractions and found SIRT3 largely in the mitochondrial fraction (Fig. 5E) and SIRT5 in the cytosolic and mitochondrial fractions (Fig. 5F).
Fig. 5. SIRT3 and SIRT5 in human lens proteins and epithelial cells.
Western blotting of the WS fraction from human lens of varying age using a monoclonal antibody against SIRT3 (A) or SIRT5 (C). The location of SIRT3 and SIRT5 are indicated by arrows. Ponceau staining of western blotted membranes for panels A and C are shown in panels B and D to demonstrate equal protein loading. S3, recombinant SIRT3 and S5, recombinant SIRT5 are indicated by dotted arrows. To identify subcellular localization of SIRT3 and SIRT5 in human lens epithelial cells, the subcellular fractions were western blotted for SIRT3 (E) and SIRT5 (F). Western blots to confirm fractionation into cytosolic, mitochondrial and nuclear fractions are shown in Fig. 4. M, molecular weight markers;
3.7. Proteins of human lens and lens epithelial cells possess depropionylase and demalonylase activities.
Western blot analysis revealed that the propionylated and malonylated lysozymes underwent depropionylation and demalonylation, respectively, upon incubation with the human lens epithelial cell lysate in the presence of NAD+ (Fig. 6A and B). Densitometric analysis of the western blots (normalized to total protein density in the Ponceau-stained membranes, shown in the right panels) showed significant, 33% and 27%, reductions in the PropK and MalK content of the respective lysozyme. However, WS proteins from the human lens, when incubated with the propionylated or malonylated lysozyme, showed no reduction in PropK and MalK content (Fig. 6C and D). In these experiments, water-soluble protein from heart tissues was used as a positive control, which significantly reduced the PropK or MalK levels of the respective lysozyme (lane 5 in all panels). These results suggest that sirtuins present in human lens epithelial cells are catalytically active (provided they have sufficient NAD+ and substrates available).
Fig. 6. SIRT3 and SIRT5 are enzymatically active in cultured human lens epithelial cells but not in human lens homogenates.
Deacylation of propionylated or malonylated lysozyme by the lens epithelial cell lysate (A and B) and WS human lens proteins (C and D) was determined by western blotting using an antibody against PropK or MalK. Deacylation of acylated lysozyme by the mouse heart extract was used as the positive control (lane 5 in all panels). Densitometric plots for the acylation levels in lysozyme are also shown. The bar graphs represent the means ± standard deviation of triplicate measurements. The Ponceau stained membrane images of respective blots are also shown to demonstrate equal protein loading. M, molecular weight markers; lane 1, acylated lysozyme alone; lane 2, WS human lens protein; lane 3, acylated lysozyme + WS human lens protein; lane 4, WS mouse heart protein; lane 5, acylated lysozyme + WS mouse heart protein. NS, non-significant; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
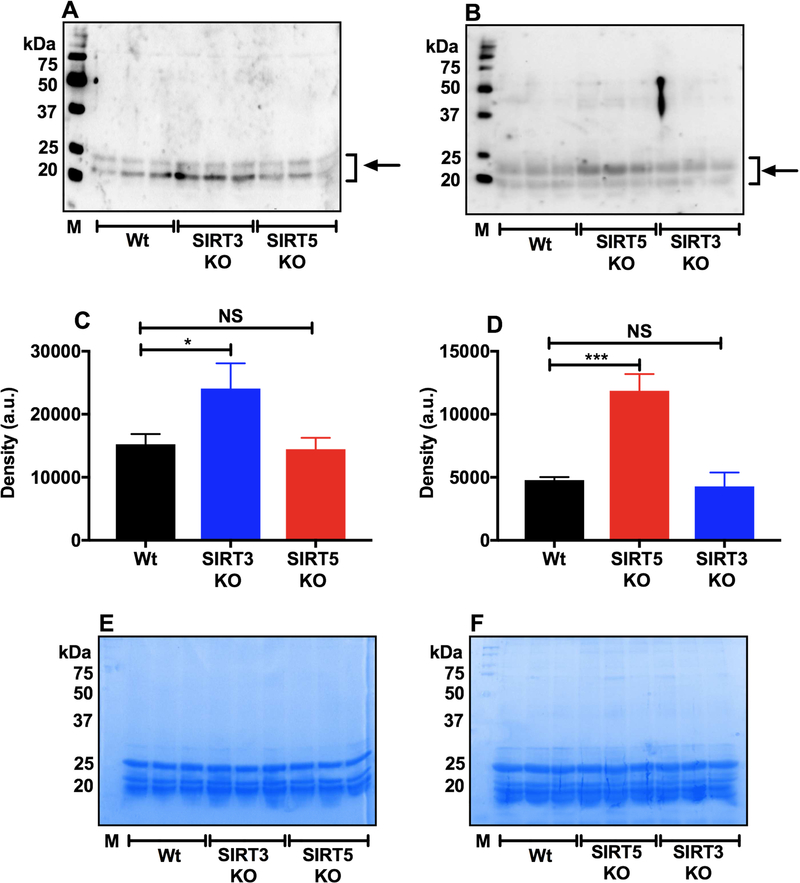
3.8. Higher levels of PropK- and MalK-modified proteins were observed in SIRT3 KO and SIRT5 KO lens cells.
We determined whether the absence of sirtuin-3 and −5 can promote malonylation and propionylation of mouse lens proteins. We found a significant increase in the levels of propionylated proteins in SIRT3 KO but not in SIRT5 KO lenses compared with WT lenses (Fig. 7A and C). The malonylated protein levels were significantly higher in SIRT5 KO lenses, but not in SIRT3 KO lenses, than in WT lenses (Fig. 7B and D). Coomassie blue staining of the SDS-PAGE gels (after transfer to the membrane) is shown in Fig. 7E and F.
Fig. 7. The absence of SIRT3 and SIRT5 increases PropK and MalK modifications in mouse lenses.
Western blotting for PropK (A) and MalK (B) in the WS fraction from WT, SIRT3 KO and SIRT5 KO mouse lenses. Densitometric plots for protein bands in panels A and B (indicated by arrows) are shown in panels C and D. SDS-PAGE (gel after transfer) stained with Coomassie stain in panels E and F show equal protein loading. The bar graphs are the means ± SD of triplicate measurements. M, molecular weight markers. NS, not significant; *p< 0.05, ***p< 0.001.
4. DISCUSSION
The goals of this study were to 1) investigate whether lysine propionylation and malonylation occurs in human lens proteins, 2) determine age-related changes in the levels of MalK and PropK in human lens proteins and 3) determine whether SIRT3 and SIRT5 are present in human lenses, whether they are functionally active and whether their absence leads to higher malonylation and propionylation levels in lens proteins.
Our study demonstrated the propionylation and malonylation of human lens proteins, both in WS and WIS proteins. Both malonylation and propionylation were predominantly observed in a protein(s) just above the 20-kDa mark in the western blots, both in the WS and WIS protein fractions, and the immunoreactivity for this protein increased with the age of the sample. Our previous studies have already demonstrated a similar predominant protein that was acetylated and succinylated just above the 20-kDa mark in western blots (Nandi et al., 2019a; Nandi et al., 2019b), which we demonstrated to be αB-crystallin. Thus, it is possible that αB-crystallin is the dominant malonylated and propionylated protein in human lenses. Although an increasing trend for MalK and PropK levels with advancing lens age was observed in the LC-MS/MS data, there was no statistical significance between age of the lens and the MalK and PropK levels.
Mass spectrometry analysis of the WS proteins from young and aged human donor lens showed lysine malonylation at K166 of αΑ-crystallin and at K90, K92 and K166 of the αB-crystallin. Similarly, lysine propionylation was observed at K70, K99 and K166 of the αΑ-crystallin and K72, K90, K92, K103, K166 and K174 of the αB-crystallin. Thus, compared to the αA-crystallin, more sites in the αB-crystallin were malonylated and propionylated. This result is possibly due to greater levels of reactive lysine residues in αB-crystallin than in αΑ-crystallin, as demonstrated in our previous study (Nandi et al., 2019b). Interestingly, a higher number of PropK sites were found in the αB-crystallin in the aged lens than in the young lens. This finding could explain the western blot results showing greater PropK levels in the older lenses than in the younger lenses. In addition to αA- and αB-crystallin, malonylation and propionylation sites were also identified in β- and γ-crystallins. Considering our previous work (Nagaraj et al., 2012; Nandi et al., 2019b), there appears to be extensive overlap between the four acyl modifications, AcK, SuccK, MalK and PropK, that we have studied thus far. For example, K92 and K166 in αB-crystallin, K44 in βA3-crystallin, K120, K121 and K172 in βB2-crystallin, K14 and K159 in γS-crystallin are the sites at which all four acyl modifications have been detected. It will be interesting to determine the relative abundance of each acyl modification at specific lysine residues and their impact on the structure and function of the proteins. We have previously shown that both acetylation and succinylation improve the chaperone activity of αB-crystallin. Whether malonylation and propionylation have a similar effect is not known and should be investigated. If acylation modifications considerably improve chaperone activity of αB - crystallin, then it could be considered an adoptive modification by lens proteins to maintain lens transparency during aging.
Lysine acylations are reversible. We previously demonstrated that SIRT3 and SIRT5 deacetylate lens proteins (Nandi et al., 2019a). Several authors have demonstrated that SIRT3 has depropionylase and SIRT5 demalonylase activities (Du et al., 2011; Fritz et al., 2013; Garrity et al., 2007). Therefore, we investigated the role of SIRT3 and SIRT5 in the demalonylation and depropionylation of lens proteins. Using SIRT3 and SIRT5 KO mice, we demonstrated that SIRT3 regulates propionylation and that SIRT5 regulates malonylation of lens proteins. There have been no reports on the expression of these two sirtuins in human lenses. Our results showed that, in human lens epithelial cells, SIRT3 and SIRT5 are both present and are catalytically active in depropionylating and demalonylating acylated lysozyme. However, we failed to detect the depropionylase or demalonylase activity of SIRT3 and SIRT5 in human lens WS proteins. This result led us to infer that depropionylase and demalonylase activity of SIRT3 and SIRT5 are active in lens epithelial cells but not in fiber cells. This finding could be due to lens epithelial cells being metabolically active, undergoing proliferation and differentiation and regulation of malonylation and propionylation could play a role in cell metabolism, proliferation and differentiation. Further work is needed to understand the importance of acylation and its regulation in lens epithelial cells.
In summary, to the best of our knowledge, this is the first report of propionylation and malonylation in human lens proteins. If acylation modifications are static in fiber cells, as suggested by our studies, then their impact on other age-associated posttranslational lysine modifications, such as glycation, and how they impact crystallin-crystallin interactions need to be investigated. The fact that lens epithelial cells have sirtuins and that they are catalytically active suggests a role for dynamic acylation modification in the functions of these cells.
Supplementary Material
Fig. S1. MalK and PropK antibodies show specific immunoreaction. Lysozyme was acylated and subjected to Western blot for MalK (A) and PropK (B) as described in the methods. Images of the Ponceau stained membrane of the respective blots show equal protein loading. 1 and a, unmodified lysozyme, 2 and c, malonylated lysozyme and 3 and b, propionylated lysozyme.
Fig. S2. Prior treatment of the MalK and PropK antibodies with acylated lysozyme reduces immunoreactivity in human lens proteins. Human lens WS from donor age 37, 43 and 55 and acylated lysozyme (malonylated and propionylated) were subjected to western blotting. Membranes were incubated with the primary antibody (for Malk or PropK) or antibodies preincubated with malonylated or propionylated lysozyme. Densitometric plots for the entire lanes normalized to total protein in Ponceau stained membranes (middle panels) are shown in the lower panels.
HIGHLIGHTS.
SIRT3 and SIRT5 are present in human lenses, and they are catalytically active in epithelial cells but not in fiber cells.
Malonylation and propionylation of lysine residues occurs in human lens proteins and the levels of MalK and PropK do not change significantly during aging.
The absence of SIRT3 and SIRT5 leads to increased propionylation and malonylation proteins in mouse lenses.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants EY028836 and EY023286 (R.H.N.) and AA022146 (K.S.F.), RPB challenge grant to the Department of Ophthalmology, University of Colorado. The authors acknowledge the Skaggs School of Pharmacy and Pharmaceutical Sciences Mass Spectrometry Core Facility, which is supported by the Colorado Clinical and Translational Sciences Institute UL-1-RRO25780, for assistance with sample analysis. We also thank the Light Microscopic Core Facility at University of Colorado for their help with confocal imaging.
Abbreviations:
- MalK
malonyllysine
- PropK
propionyllysine
- WS
water-soluble lens protein
- WIS
water-insoluble, solubilized lens protein
- PTM
posttranslational modification
- SOD2
superoxide dismutase-2
- DTT
dithiothreitol
- WT
wild type
- KO
knockout
- HDAC
histone deacetylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare that there are no competing interests associated with the manuscript.
REFERENCES
- Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH, 2017. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 16, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning U, Morales-Rodriguez F, Kalucka J, Goveia J, Taverna F, Queiroz KCS, Dubois C, Cantelmo AR, Chen R, Loroch S, Timmerman E, Caixeta V, Bloch K, Conradi LC, Treps L, Staes A, Gevaert K, Tee A, Dewerchin M, Semenkovich CF, Impens F, Schilling B, Verdin E, Swinnen JV, Meier JL, Kulkarni RA, Sickmann A, Ghesquiere B, Schoonjans L, Li X, Mazzone M, Carmeliet P, 2018. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab 28, 866–880e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BJ, Lam TC, Liu LQ, To CH, 2017. Post-translational modifications and their applications in eye research (Review). Mol Med Rep 15, 3923–3935. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sprung R, Tang Y, Ball H, Sangras B, Kim SC, Falck JR, Peng J, Gu W, Zhao Y, 2007. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol Cell Proteomics 6, 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu JM, 2005. The Sir 2 family of protein deacetylases. Curr Opin Chem Biol 9, 431–440. [DOI] [PubMed] [Google Scholar]
- DiMauro MA, Nandi SK, Raghavan CT, Kar RK, Wang B, Bhunia A, Nagaraj RH, Biswas A, 2014. Acetylation of Gly1 and Lys2 promotes aggregation of human gammaD-crystallin. Biochemistry 53, 7269–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H, 2011. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Hu H, Hua C, Du K, Wei T, 2018. Tissue distribution, subcellular localization, and enzymatic activity analysis of human SIRT5 isoforms. Biochem Biophys Res Commun 503, 763–769. [DOI] [PubMed] [Google Scholar]
- Fritz KS, Green MF, Petersen DR, Hirschey MD, 2013. Ethanol metabolism modifies hepatic protein acylation in mice. PLoS One 8, e75868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA, 1999. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun 260, 273–279. [DOI] [PubMed] [Google Scholar]
- Frye RA, 2000. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273, 793–798. [DOI] [PubMed] [Google Scholar]
- Galvan-Pena S, Carroll RG, Newman C, Hinchy EC, Palsson-McDermott E, Robinson EK, Covarrubias S, Nadin A, James AM, Haneklaus M, Carpenter S, Kelly VP, Murphy MP, Modis LK, O’Neill LA, 2019. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat Commun 10, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity J, Gardner JG, Hawse W, Wolberger C, Escalante-Semerena JC, 2007. N-lysine propionylation controls the activity of propionyl-CoA synthetase. J Biol Chem 282, 30239–30245. [DOI] [PubMed] [Google Scholar]
- Gattner MJ, Vrabel M, Carell T, 2013. Synthesis of epsilon-N-propionyl-, epsilon-N-butyryl-, and epsilon-N-crotonyl-lysine containing histone H3 using the pyrrolysine system. Chem Commun (Camb) 49, 379–381. [DOI] [PubMed] [Google Scholar]
- Grevengoed TJ, Klett EL, Coleman RA, 2014. Acyl-CoA metabolism and partitioning. Annu Rev Nutr 34, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, 2011. Old enzymes, new tricks: sirtuins are NAD(+)-dependent de-acylases.Cell Metab 14, 718–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Zhao Y, 2015. Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol Cell Proteomics 14, 2308–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Smith CL, Smith AC, Robinson AJ, Hoogewijs K, Murphy MP, 2018. The Causes and Consequences of Nonenzymatic Protein Acylation. Trends Biochem Sci 43, 921–932. [DOI] [PubMed] [Google Scholar]
- Kebede AF, Nieborak A, Shahidian LZ, Le Gras S, Richter F, Gomez DA, Baltissen MP, Meszaros G, Magliarelli HF, Taudt A, Margueron R, Colome-Tatche M, Ricci R, Daujat S, Vermeulen M, Mittler G, Schneider R, 2017. Histone propionylation is a mark of active chromatin. Nat Struct Mol Biol 24, 1048–1056. [DOI] [PubMed] [Google Scholar]
- Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y, 2010. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5, e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr., Weissman S, Verdin E, Schwer B, 2007. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27, 8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, Biswas A, 2012. Acetylation of alphαΑ-crystallin in the human lens: effects on structure and chaperone function. Biochim Biophys Acta 1822, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Huang R, Nandi SK, Wang B, Padmanabha S, Santhoshkumar P, Filipek S, Biswas A, Nagaraj RH, 2013a. Acetylation of lysine 92 improves the chaperone and anti-apoptotic activities of human alphaB-crystallin. Biochemistry 52, 8126–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Oya-Ito T, Nagaraj RH, 2013b. The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human alpha-crystallin. Biochim Biophys Acta 1832, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, Nagaraj RH, 2013c. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem 288, 13022–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L, 2009. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi SK, Nahomi RB, Harris PS, Michel CR, Fritz KS, Nagaraj RH, 2019a. The absence of SIRT3 and SIRT5 promotes the acetylation of lens proteins and improves the chaperone activity of alpha-crystallin in mouse lenses. Exp Eye Res 182, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi SK, Rakete S, Nahomi RB, Michel C, Dunbar A, Fritz KS, Nagaraj RH, 2019b. Succinylation Is a Gain-of-Function Modification in Human Lens alphaB-crystallin. Biochemistry 58, 1260–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, He W, Verdin E, 2012. Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease. J Biol Chem 287, 42436–42443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Shuai L, Zhu M, Liu P, Xie ZF, Jiang S, Jiang HW, Li J, Zhao Y, Li JY, Tan M, 2017. The Landscape of Histone Modifications in a High-Fat Diet-Induced Obese (DIO) Mouse Model. Mol Cell Proteomics 16, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y, 2011. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics 10, M111012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Thorn K, Schroeder T, Wang L, Theis FJ, Marr C, Navab N, 2017. A BaSiC tool for background and shading correction of optical microscopy images. Nat Commun 8, 14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW, 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA, Wolberger C, Schramm VL, Boeke JD, 2006. The biochemistry of sirtuins. Annu Rev Biochem 75, 435–465.. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Wang F, Stieren E, Tong Q, 2005. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280, 13560–13567. [DOI] [PubMed] [Google Scholar]
- Smuda M, Henning C, Raghavan CT, Johar K, Vasavada AR, Nagaraj RH, Glomb MA, 2015. Comprehensive analysis of maillard protein modifications in human lenses: effect of age and cataract. Biochemistry 54, 2500–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinon E, Hang HC, 2015. Chemical reporters for exploring protein acylation. Biochem Soc Trans 43, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL, 2006. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res 5, 2554–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanshole LV, Cherepanov IV, Snytnikova OA, Yanshole VV, Sagdeev RZ, Tsentalovich YP, 2013. Cataract-specific posttranslational modifications and changes in the composition of urea-soluble protein fraction from the rat lens. Mol Vis 19, 2196–2208. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. MalK and PropK antibodies show specific immunoreaction. Lysozyme was acylated and subjected to Western blot for MalK (A) and PropK (B) as described in the methods. Images of the Ponceau stained membrane of the respective blots show equal protein loading. 1 and a, unmodified lysozyme, 2 and c, malonylated lysozyme and 3 and b, propionylated lysozyme.
Fig. S2. Prior treatment of the MalK and PropK antibodies with acylated lysozyme reduces immunoreactivity in human lens proteins. Human lens WS from donor age 37, 43 and 55 and acylated lysozyme (malonylated and propionylated) were subjected to western blotting. Membranes were incubated with the primary antibody (for Malk or PropK) or antibodies preincubated with malonylated or propionylated lysozyme. Densitometric plots for the entire lanes normalized to total protein in Ponceau stained membranes (middle panels) are shown in the lower panels.