Figure 6. Model for the regulation of PilB signaling activity by cdG and ADP.
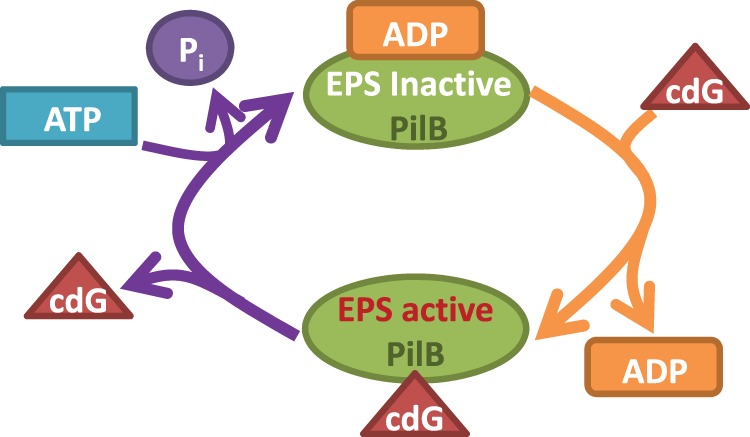
PilB is inactive in EPS signaling when it is associated with ADP. In this ADP-bound state, however, PilB has high affinity for cdG and it has a high probability to bind cdG. The binding of cdG, in turn, results in a decrease in the affinity of PilB for ADP and the release of ADP leads to the PilB–cdG complex to its active EPS-signaling conformation. The dissociation of cdG inactivates PilB in EPS signaling and may simultaneously facilitates the ATP hydrolysis cycle by PilB. The left side of this model is functionally analogous to the conversion of a G-protein from a GDP-bound and inactive signaling state to GTP-bound and active state which is catalyzed by GEF.
