Highlights
-
•
Multiorgan failure (MOF) might produce long-term neurological and cognitive sequelae.
-
•
Functional MRI revealed abnormal default mode network (DMN) connectivity in MOF.
-
•
DMN connectivity 6 months after MOF was associated with clinical severity during ICU .
-
•
DMN connectivity was associated with attention and visual-constructional abilities.
-
•
Resting-state fMRI is a potential tool for predicting long-term deficits in MOF.
Keywords: Multiorgan failure, Multiple organ dysfunction syndrome, Functional connectivity, Resting state, Hyper-connectivity, Default Mode Network, Neuropsychological Evaluation
Abstract
Multiorgan failure (MOF) is a life-threating condition that affects two or more systems of organs not involved in the disorder that motivates admission to an Intensive Care Unit (ICU). Patients who survive MOF frequently present long-term functional, neurological, cognitive, and psychiatric sequelae. However, the changes to the brain that explain such symptoms remain unclear.
Objective
To determine brain connectivity and cognitive functioning differences between a group of MOF patients six months after ICU discharge and healthy controls (HC).
Methods
22 MOF patients and 22 HC matched by age, sex, and years of education were recruited. Both groups were administered a 3T magnetic resonance imaging (MRI), including structural T1 and functional BOLD, as well as a comprehensive neuropsychological evaluation that included tests of learning and memory, speed of information processing and attention, executive function, visual constructional abilities, and language. Voxel-based morphometry was used to analyses T1 images. For the functional data at rest, functional connectivity (FC) analyses were performed.
Results
There were no significant differences in structural imaging and neuropsychological performance between groups, even though patients with MOF performed worse in all the cognitive tests. Functional neuroimaging in the default mode network (DMN) showed hyper-connectivity towards sensory-motor, cerebellum, and visual networks. DMN connectivity had a significant association with the severity of MOF during ICU stay and with the neuropsychological scores in tests of attention and visual constructional abilities.
Conclusions
In MOF patients without structural brain injury, DMN connectivity six months after ICU discharge is associated with MOF severity and neuropsychological impairment, which supports the use of resting-state functional MRI as a potential tool to predict the onset of long-term cognitive deficits in these patients. Similar to what occurs at the onset of other pathologies, the observed hyper-connectivity might suggest network re-adaptation following MOF.
1. Introduction
Multiorgan Failure (MOF), also known as Multiple Organ Dysfunction Syndrome (MODS), is a potentially reversible physiological disorder that affects two or more organ systems not involved in the condition that motivates admission to an Intensive Care Unit (ICU). MOF arises after a potentially life-threatening physiologic insult and it is currently the most common cause of death in patients admitted to an ICU (Marshall, 2001).
During the last years, most attention regarding the prevention and treatment of MOF has focused on patient survival during ICU stay. However, there is now sufficient evidence that MOF survivors usually present neurological, cognitive, and neuropsychiatric manifestations in the months and years after discharge from the ICU (Ehlenbach, 2010; Iwashyna et al., 2010; Latronico and Bolton, 2011; Wolters et al., 2013). Specifically, it has been shown that 34% of MOF patients had global cognition scores 1.5 SD below the mean of population at 12 months after ICU discharge (similar to moderate traumatic brain injury), and 24% had scored 2 SD below the mean of the population (similar to mild Alzheimer's disease) (Pandharipande et al., 2013). The results from previous research have led to hypothesize that microstructural injury and abnormal brain functional connectivity could be responsible for the appearance of these deficits.
On the other hand, some clinical variables related to the severity of the MOF, such as advanced age, hypoxemia, hypotension, the number of sedation days, and of days with delirium (Ely et al., 2004; Girard et al., 2010; Gunther et al., 2012; Pandharipande et al., 2013) have been associated with the presence of cognitive deficits after ICU discharge. However, in cases where no brain damage is present, the mechanisms underlying the long-term neurological and cognitive deficits after MOF are not well understood. In particular, whether or not those patients present the immune system's hyper-activation that can propagate to the central nervous system is still unknown, and the research regarding the cognitive impairment in these patients is almost inexistent. Indeed, it is of crucial interest to understand the pathophysiological mechanisms of these brain functioning aftermaths, in order to define better protocols to improve patient's quality of life (Needham et al., 2012).
Even though some studies have shown that different RSNs are altered in pathological conditions like traumatic brain injury (Diez et al., 2017), schizophrenia (Karbasforoushan and Woodward, 2012; Woodward et al., 2011), epilepsy (Liao et al., 2010), Alzheimer's Disease (Binnewijzend et al., 2012; Diez et al., 2015; Greicius et al., 2004; Li et al., 2002; Rombouts et al., 2005), and healthy aging (Bonifazi et al., 2018) today, there is a lack of research studies that evaluated the brain connectivity of RSNs and its relationship with the cognitive function of these patients after MOF. Thus, the aims of this study are: 1) To determine differences in brain connectivity and cognitive functioning between a group of MOF patients six months after ICU discharge and Healthy Controls (HC), and 2) To explore if there is a relationship between brain connectivity and cognitive function in these patients after six months of MOF.
2. Materials and methods
2.1. Participants, design, and clinical variables
A cross-sectional study was performed based on neuropsychological and brain MRI of 22 patients that suffered MOF and had been treated at the ICU of the Cruces University Hospital (Bilbao, Spain). The same evaluation was also performed to 22 HC , recruited in the vicinity of the Cruces University Hospital and matched with patients by age, sex, and years of education. The evaluation of MOF patients was performed six months after ICU discharge in order to avoid the individual effects of administered drugs during ICU admission. During ICU stay a set of basic physiological variables were collected in MOF patients, including mean blood pressure, mean arterial partial oxygen pressure (pO2), and days of sedation. These variables were calculated from the raw data stored in IntelliSpace Critical Care & Anesthesia Information (ICCA) platform (Koninklijke Philips N.V.). The severity of MOF was assessed by the Sequential Organ Failure Assessment (SOFA) score, that used to quantify the extent of organ dysfunction in patients after MOF (Jones et al., 2009), assigns to each of the following six systems respiratory, coagulatory, liver, cardiovascular, renal, and neurologic a score from 0 (normal) to 4 (high degree of dysfunction/failure), so the total score ranges from 0 to 24. Furthermore, right after ICU discharge, the overall cognitive status of patients was evaluated with the Mini-Mental State Examination (MMSE).
The inclusion criteria for MOF patients were: a) were between 18 and 65 years of age; b) had a MOF due to respiratory failure, cardiogenic shock, or septic shock; c) not having had structural brain injury detected in T1 (no T1-BD); d) SOFA score ≥ 4 for at least 48 h during ICU admission, implying an associated mortality rate of at least 20% (Ferreira et al., 2001); e) MMSE score ≥ 23 (Folstein et al., 1975); and f) were able to read and write at the time of evaluation.
The inclusion criteria for the HC were: a) were between 18 and 65 years of age; b) MMSE score ≥ 23; c) scored ≤ 4 on the Patient Health Questionnaire–9 for assessing depression; and d) were able to read and write at the time of evaluation.
The exclusion criteria for MOF patients were: a) history of developmental problems or learning disabilities prior to admission to the ICU; b) cerebral hypoxia during ICU admission; c) history of neurological or psychiatric conditions; d) chronic organ injury that might alter functional connectivity (Li et al., 2016); e) regular use medications that may impact cognitive functioning; f) history of daily consumption and/or use of an illicit substance; g) severe visual and/or hearing deficit at the time of the evaluation; and h) contraindications for magnetic resonance imaging (i.e., morbid obesity, pacemaker, metal prostheses, or pregnancy).
The exclusion criteria for the HC were: a) history of neurological or psychiatric conditions; b) history of developmental problems or learning disabilities; c) regular use medications that may impact cognitive functioning; d) daily consumption and/or use of an illicit substance; e) severe visual and/or hearing deficit; and f) contraindications for magnetic resonance imaging (i.e., morbid obesity, pacemaker, metal prostheses, or pregnancy).
The study was approved by the local Ethical Committee at the Cruces University Hospital (Code CEIC E16/52). All participants gave their written informed consent prior to their participation in the study, which was performed in accordance with the Helsinki Declaration.
2.2. Neuropsychological evaluation
Both MOF patients and HC were administered a comprehensive neuropsychological evaluation that includes the following tests:
2.2.1. Rey-Osterrieth Complex Figure (ROCF)
The ROCF is a test that assesses visual perception, visual-spatial constructional ability, and visual memory. The test is composed of two parts (Rey, 2009): the copy and the memory task. During the copy task, participants are asked to draw-copy a complex figure whilst looking to a template, which contains 18 different elements. During the memory task (conducted 3 min after the copy part), participants are requested to draw the same figure they copied before but without looking to it anymore (remembering). The maximum and minimum scores for each task (copy and memory) are respectively 36 and 0. ROCF takes around 10 min to be completed.
2.2.2. Verbal Fluency Test (VFT)
The verbal fluency test assesses an individual's capacity for complex cognitive functioning, such as language and executive functioning (Strauss et al., 2006). Different modalities of VFT exist, but in this study, it was used the two more common, namely, the phonological and semantic VFT. In the phonological VFT, the participant is required to produce in 60 s as many words as possible, all of them starting with the same initial letter (here, M, R, and P were used). In the semantic VFT, the participant is required to produce in 60 s as many words as possible, all of them belonging to a particular category (here, animals and fruits were used). For administration and scoring, the protocol explained in (Olabarrieta-Landa et al., 2017) was followed. VFT takes around 5 min to be completed.
2.2.3. Hopkins Verbal Learning Test-Revised (HVLT-R)
The HVLT-R is a test to measure verbal learning and memory (Benedict et al., 1998). The test consists of a 12-item word list that is read to participants on three successive learning trials. After reading the final word, the participant is asked to recall as many items as possible in any order (Total Recall). After 20–25 min, patients are requested to recall as many words as possible (Delayed Recall). Total Recall score ranges between 0 and 36 and Delayed recall from 0 to 12. HVLT-R takes around 10 min to be completed.
2.2.4. Stroop color and word test (Stroop)
The Stroop test is a measured of executive functioning of selective attention, cognitive flexibility, cognitive inhibition, and information processing speed (Golden, 2010). The test consists of three parts, each with 100 stimuli randomly organized into five columns. The first sheet (Word condition) is formed by the words “Red”, “Green,”, and “Blue” colored in black, and the participant is requested to read aloud, as quick as possible, the columns from left to right. The second sheet (Color condition) is formed by groups of four Xs (“XXXX”) colored in blue, green, and red, and the task is to name the ink color of each stimulus. Finally, the last sheet (Word-Color condition) consists of the three words of the first printed page in the colors of the second, with words being incongruent with the color of the ink. The task is to name the ink color, inhibiting the reading of the word. Each condition of the test (Word, Color, Word-Color) takes 45 s to complete and the score is the number of correctly named elements in each condition. Stroop takes around 3 min to be completed.
2.2.5. Modified Wisconsin Card Sorting Test (M-WCST)
This test was used to measure global executive functioning, that is, the use of abstract reasoning, strategic planning, organized searching, mental flexibility, and impulse control (Schretlen, 1997). The test consists of four stimulus cards and 48 response cards. Each card varies in shape (cross, circle, triangle, or star), color (red, blue, yellow, or green), and number (one to four). Participants are asked to classify correctly the stimulus cards according to certain rules. The test continues until all six categories are classified or until the whole volume has been used. The test allows for the calculation of the number of categories , perseveration errors, and total errors. M-WCST takes around 15 min to be completed.
2.2.6. Trail Making Test (TMT A-B)
This test assesses psychomotor speed, visual scanning, attention, sequencing, and mental flexibility (Reitan and Wolfson, 1985). The test consists of two parts: TMT-A, during which the participant is requested to connect with a line 25 numbers in ascending order, which are circled and randomly distributed on a sheet of paper. In the TMT-B, the participant is asked to connect numbers and letters in an alternative way (1-A, 2-B, 3-C, . . .). The total score in each task is the needed time in seconds to complete the task. The time limit for TMT-A is 100 s and 300 s for TMT-B. TMT takes around 10 min to be completed.
2.2.7. Brief Test of Attention (BTA)
The BTA is a test to measure auditory-divided attention (Schretlen et al., 1996) and consists of two equivalent forms that are administered one after the other (forms N and L). Each form contains a list of letter-numeric strings increasing in length from 4 to 18 characters. In the first list (form N), the participant is asked to count how many numbers have been presented, while discarding the letters. The exact same items are presented on the second list (form L), but the participant is asked to count the number of letters presented while ignoring the numbers. One point is awarded for each correct answer, with each list ranging from 0 to 10. The number of correctly identified items is summed across both forms, with total scores ranging between 0 and 20. BTA takes around 5 min to be completed.
2.2.8. Symbol Digit Modalities Test (SDMT)
SDMT was created to assess the cognitive functioning of attention, motor speed, and visual scanning (Smith, 2013). The test is composed of a key with two rows, nine stimulus symbols in the upper one and matched numbers (1–9) in the row below. Participants are required to write, as fast as possible, the correct number under the corresponding symbol in 90 s. The final score is the number of correct completed substitutions, with a maximum score of 110. For this study, written administration was used.
2.2.9. Boston Naming Test (BNT)
BNT is a test created to assess language abilities including naming and word retrieval (Kaplan et al., 2005). The test consists of 60 pictures, which are presented in order of increasing difficulty to the participant with the aim of naming them. The total score is the sum of correct spontaneous answers (answers with no cues) plus correct answers (followed by a semantic clue). BNT takes around 15 min to be completed.
2.3. Imaging acquisition
The brain MRIs were acquired in a Philips 3-Tesla Achieva Dstream MRI scanner with a 32-channel head coil and included the following sequences:
2.3.1. Anatomical data
High resolution T1 images were acquired with a 3D Turbo Field Echo (TFE): repetition time TR = 7.4 ms, echo time TE = 3.4 ms, inversion time IT = 850 ms, voxel size = 1.1 × 1.1 × 1.2 mm3, slice thickness = 1.2 mm, field of view FOV = 250 × 250 mm2, 300 contiguous sagittal slices covering the entire brain and brainstem.
2.3.2. Resting state functional data
A session with a total duration of 7.40 min was acquired, using SENSE (with a factor of 2.2) and the following parameters: 214 whole-brain gradient echo-planar images with TR/TE = 2100/27 ms, FOV = 240 × 240 mm2, voxel size = 3 × 3 × 3 mm3, 80 × 80 matrix, slice thickness of 3 mm, 45 axial slices, interleaved in ascending order.
2.4. Imaging analyses
2.4.1. Structural abnormalities
Voxel-based morphometry (VBM) was applied with FSL-VBM (Douaud et al., 2007), FSL version 6.0.1, an optimized VBM protocol carried out with FSL tools. First, by using the structural images, skull was removed and gray matter was segmented before being registered to the MNI152 standard space with non-linear registration. The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific gray matter template. Second, all native gray matter images were non-linearly registered to this study-specific template and "modulated" to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated gray matter images were then smoothed with an isotropic Gaussian kernel of full width at half maximum of 9.42 mm (σ = 4 mm). Final images were used for group comparison.
2.4.2. Functional preprocessing
The rs-fMRI images were preprocessed with FSL 6.0.1 and AFNI 19.3.00 (https://afni.nimh.nih.gov/) similar to that used in previous work (Camino-Pontes et al., 2018; Rasero et al., 2017). First, slice-time correction was applied to the fMRI dataset. Next, each volume was aligned to the middle volume to correct for head motion artifacts using MCFLIRT (incorporated in FSL). After intensity normalization, the effect of the following cofounding factors was removed: movement time-courses, average cerebrospinal fluid (CSF), and white matter (WM) signals, together with the linear and quadratic trends. Finally, a band-pass filter was used within the 0.01–0.08 Hz band (Cordes et al., 2001). Next the functional data was spatially normalized to the MNI152 brain template, with a voxel size of 3 × 3 × 3 mm3 and all voxels were spatially smoothed with a 6 mm full width at half maximum (FWHM) isotropic Gaussian kernel. Finally, scrubbing was performed, by which time points with frame-wise displacement (FD) greater than 0.5 were removed together one frame before and two frames after (Power et al., 2014), which is a more conservative strategy ensuring that movements artifacts are sufficiently removed.
2.4.3. Functional connectivity and maps of connectivity strength
Following (Smith et al., 2009), a mask for different RSNs was created by defining voxels with z-score value satisfying z 〈 −3 or z 〉 3. In particular, the following masks were built: default mode, cerebellum, executive control, fronto-parietal, sensory-motor, auditory, lateral visual, medial visual and occipital pole. These masks were used to calculate strength brain maps of connectivity from all voxels to each specific RSN.
After preprocessing of the functional data, each subject was represented by 50125 time series (one per voxel), each one with T = 214 time points. FC matrices with dimension of 50125×50125 were obtained per each subject by calculating the pairwise Pearson correlation of the time series. Brain maps of strength were obtained by summing the functional connectivity (FC) matrix over either rows or columns, as FC is a symmetrical matrix.
In addition to the strength maps obtained from the all-to-all FC matrix (the one with dimension 50125 × 50125), it was also obtained other FC matrices by calculating the correlations of all voxels in the brain to those ones belonging to each specific RSN mask, thereby resulting in rectangular matrices with dimension 50125 x [RSN Size]. The strength maps were calculated by summing the dimension of the specific RSNs in the rectangular matrix. Following this procedure, the following matrices were calculated: all-to-DMN, all-to-cerebellum, all-to-executive-control, all-to-fronto-parietal, all-to-sensory-motor, all-to-auditory, all-to-lateral-visual, all-to-medial-visual and all-to-occipital-pole matrices. Thus, together with the all-to-all matrix, 10 different strength maps were calculated for each subject for the different group comparison analyses.
2.4.4. Labelling of anatomical regions
The anatomical characterization for each brain map was done using the automated anatomical labeling (AAL) brain atlas (Tzourio-Mazoyer et al., 2002), and as a consequence, the anatomical labels used in this work follow the ones existing in the AAL atlas.
2.5. Statistical and data analyses
2.5.1. Separability of group FC matrices measured by the signal to noise ratio (SNR)
Based on the signal-detection theory, the separability of the FC connectivity strength matrices was evaluated for the MOF and HC groups using the signal to noise ratio (SNR) by using the formula where µi and σi2 represent the mean and variance of group i = 1, 2, respectively (Cortes et al., 2010). Therefore, the higher the SNR, the larger the separability of the two distributions. Furthermore, μHC > μMOF situations indicated hypo-connectivity, rather μMOF > μHC showed hyper-connectivity.
2.5.2. Group differences in neuropsychological performance
Differences in each neuropsychological test was assessed by two-sample t-test implemented in Matlab 2016b (The MathWorks, Inc.). Effect size was also assessed by calculating the Cohen distance.
2.5.3. Multiple comparisons correction
For any of the image analyses, statistics was assessed independently for all the number of voxels. To correct for multiple comparisons, it was used the cluster-based thresholding method implemented in the function 3dClustSim of AFNI 19.3.00, ensuring that each voxel within a significant cluster satisfied simultaneously that its p-value was smaller than 0.05 and had a false positive rate (FPR) smaller than 0.05. The noise volume was simulated assuming the auto-correlation function (ACF) followed a mixed-model of the form: , where a,b,c were estimated by 3dFWHMx of AFNI.
2.5.4. Hypotheses contrasts
Two different contrasts were used for comparison of the brain maps: [1 −1] (MOF > HC) and [−1 1] (MOF < HC). For the association between the all-to-DMN strength maps and the clinical and neuropsychological scales, the contrast [0 1] was used, only taking into consideration the group of patients.
3. Results
General clinical features of MOF patients are presented in Table 1 and demographic variables for patients and HC are presented in Table 2. Patients had in average 51.8 years of age (range 31–64 years) and 13.9 years of education (range 6 to 23 years), and 54.5% were males. There were no significant differences in terms of age, sex distribution, or years of education between HC and MOF patients. During ICU admission the mean SOFA score of patients was 7.1 (range 4 to 16). The causes of MOF in order of frequency were respiratory insufficiency [ten patients (45.5%)], septic shock [six patients (27.3%)], cardiogenic shock [five patients (22.7%)], and septic and cardiogenic shock [one case (4.5%)]. Right after ICU discharge mean MMSE of MOF patients was 29.05 (SD: 1.40).
Table 1.
Clinical data for MOF patients. Mean values of different variables are calculated and standard deviation are given in brackets. * Number of participants who had these data available. ** Acquired after ICU discharge. All other variables were obtained during ICU stay.
| Clinical Variables, units | Value | n* |
|---|---|---|
| MMSE, total score ** | 29.05 (1.40) | 22 |
| SOFA, total score | 7.14 (4.04) | 22 |
| Mean blood pressure, mm Hg | 84.19 (6.63) | 16 |
| Mean partial pressure of arterial oxygen (pO2), mm Hg | 102.15 (17.14) | 10 |
| Sedation, days | 10.63 (12.01) | 16 |
| Acquisition time from ICU discharge, months | 6.80 (1.30) | 22 |
| Cause of MOF n (%) | 22 | |
| Septic Shock | 6 (27.27) | |
| Respiratory Insufficiency | 10 (45.45) | |
| Cardiogenic Shock | 5 (22.72) | |
| Septic Shock & Cardiogenic Shock | 1 (4.50) |
Table 2.
Demographic characteristics and neuropsychological scores of HC and MOF patients: Mean values of different variables are calculated and standard deviation are given in brackets. Neuropsychological evaluations assessed cognitive performance in four different domains: 1) Learning and memory - Rey-Osterrieth Complex Figure (ROCF) Copy and Memory, Hopkins Verbal Learning Test-Revised (HVLT-R) Recall and Delayed; 2) Speed of information processing and attention - Stroop Word and Color, Trail Making Test parts A and B (TMT A-B), Brief Test of Attention (BTA), Symbol Digit Modalities Test (SDMT); 3) Executive functioning - Stroop Word and Color, Modified Wisconsin Card Sorting Test (M-WCST Categories, Perseveration and Total Errors; 4) Language - Verbal Fluency Test (VFT) and Boston Naming Test (BNT). In general, for all the tests in this Table the higher scores reflect better performance (except for the M-WCST Perseveration, M-WCST Total Errors, TMT-A and TMT-B, where higher scores reflect a worse performance). Note that, in general MOF performed worse than HC, yet none of the tests provided significant group differences. * Three MOF patients and three HC did not consent to have the neuropsychological evaluation.
| Variables, units | Control | Patients | t | p | Effect Size |
|---|---|---|---|---|---|
| Demographics | |||||
| n | 22 | 22 | |||
| Age, years | 53.55 (7.97) | 51.77 (8.16) | 0.73 | .47 | .22 |
| Males, n (%) | 11 (50.00) | 12 (54.55) | 0.09 | .76 | −0.09 |
| Education, years | 13.64 (5.50) | 13.86 (5.10) | −0.14 | .89 | −0.04 |
| Neuropsychological assessment | |||||
| n | 19* | 19* | |||
| Learning and Memory | |||||
| ROCF-Copy | 33.05 (2.63) | 32.34 (4.11) | 0.63 | .53 | .21 |
| ROCF-Memory | 17.50 (5.14) | 16.95 (5.98) | 0.31 | .76 | .10 |
| HVLT-R Recall | 24.79 (3.72) | 23.52 (5.93) | 0.79 | .44 | .26 |
| HVLT-R Delayed | 8.63 (1.64) | 7.63 (2.67) | 1.39 | .17 | .45 |
| Speed of Information Processing and Attention | |||||
| Stroop Word | 102.47 (14.14) | 94.17 (23.10) | 1.33 | .19 | .43 |
| Stroop Color | 73.95 (8.61) | 65.67 (16.45) | 1.93 | .06 | .63 |
| TMT-A | 35.32 (12.75) | 37.21 (16.54) | −0.40 | .70 | −0.13 |
| TMT-B | 77.89 (40.48) | 85.89 (57.04) | −0.50 | .62 | −0.16 |
| BTA | 17.21 (2.10) | 15.63 (3.92) | 1.55 | .13 | .50 |
| SDMT | 50.58 (12.47) | 45.28 (12.22) | 1.31 | .20 | .43 |
| Executive Functioning | |||||
| Stroop Word-Color | 41.47 (8.06) | 37.77 (8.67) | 1.34 | .19 | .44 |
| M-WCST Categories | 4.63 (1.95) | 4.47 (1.54) | 0.27 | .78 | .09 |
| M-WCST Preservation | 3.42 (4.49) | 4.68 (4.02) | −0.91 | .37 | −0.30 |
| M-WCST Total Errors | 12.16 (11.41) | 12.47 (8.64) | −0.10 | .92 | −0.03 |
| Language | |||||
| Verbal Fluency | 83.11 (17.80) | 77.63 (17.65) | 0.95 | .35 | .31 |
| Boston | 53.05 (5.15) | 52.89 (6.19) | 0.09 | .93 | .03 |
When the neuropsychological scores for MOF at ICU discharge were compared with those for the HC (Table 2), MOF patients generally performed worse in all cognitive domains, although no significant differences existed for any of the tests. Nevertheless, the worst performance of MOF patients, corresponding to a higher effect size (ES), occurred for Stroop Color (ES=0.63), HVLT-R Delayed (ES=0.45), and BTA (ES=0.50).
In relation to the structural integrity of the brain quantitatively tested with voxel-based morphometry on T1 MRI sequences, significant differences between MOF patients and HC were not found. Figure S1 shows three axial slices (z = 75) illustrating ventricle space, together with white and gray matter for three arbitrarily chosen MOF patients. Although group differences are not easily detected in T1 after visual inspection, quantitative comparison with voxel-based morphometry did not provide either any significant group differences for any of the contrasts [1 −1] or [−1 1], respectively, MOF > HC and MOF < HC. Therefore, both qualitative and quantitative standard methods for analyzing structural imaging showed no structural damage in MOF patients.
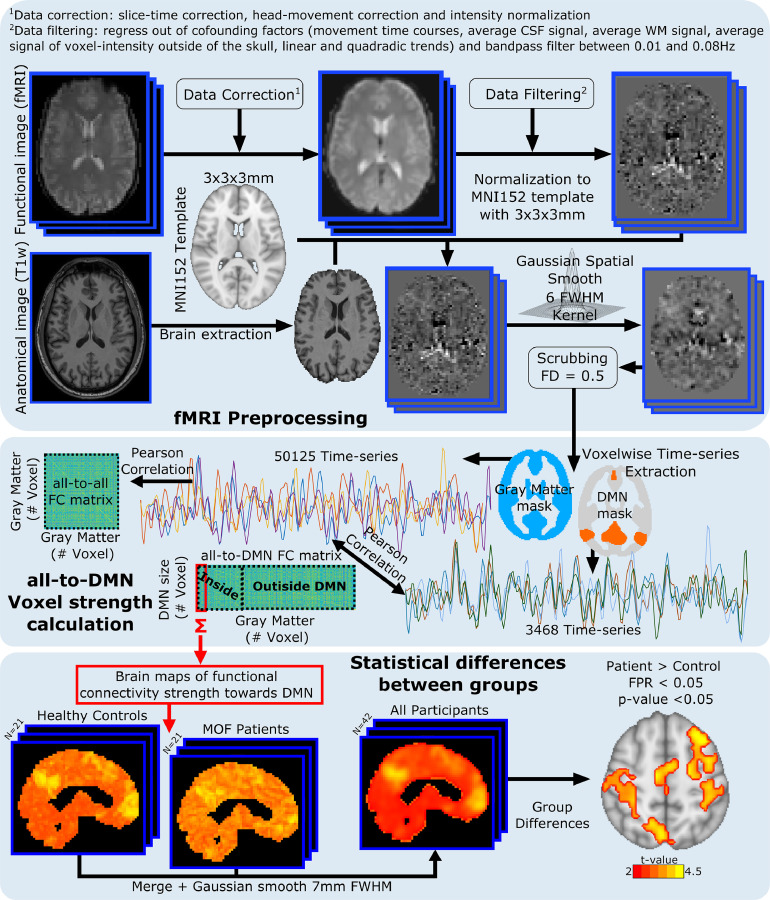
Regarding the analysis of the functional integrity of the brain tested with rs-fMRI, group differences were assessed in strength maps obtained from FC matrices. Fig. 1 illustrates the method for the all-to-DMN FC matrix, but the same analysis was repeated for the following matrices: all-to-all, all-to-cerebellum, all-to-executive-control, all-to-frontoparietal, all-to-sensory-motor, all-to-auditory, all-to-lateral-visual, all-to-medial-visual and all-to-occipital-pole.
Fig. 1.
General pipeline to analyze functional data. Double acquisition is needed: High-resolution anatomical images (T1) and functional images at rest. Following state-of-the-art pipeline of neuroimage preprocessing, time-series of the blood oxygenation level dependent (BOLD) signal were obtained for each voxel. Different functional connectivity (FC) matrices were calculated: all-to-all (accounting for the correlations from all voxels to all voxels); all-to-DMN (from all voxels to only those ones belonging to the DMN, illustrated in this figure); all-to-cerebellum; all- to-executive-control; all-to-fronto-parietal; all-to-sensory-motor; all-to-auditory; all-to-medial-visual; all-to-lateral-visual and all-to-occipital-pole. Summing along the shorter dimension in the rectangular FC matrix enabled to build strength brain maps for each subject, finally used for performing group comparisons (see methods for details).
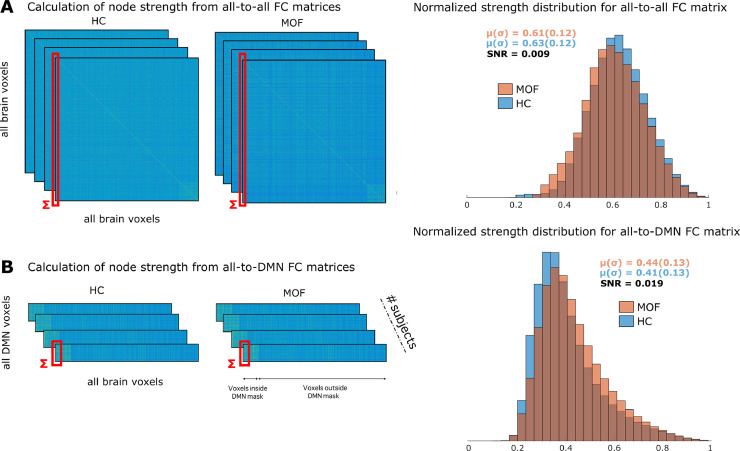
Fig. 2 illustrates the probability distribution of strength values calculated for the all-to-all (Fig. 2A) and all-to-DMN (Fig. 2B) matrices for the two groups: HC (colored in blue) and MOF (in red). The separability of the two distributions had a relative increase of 311%, as the SNR was equal to 0.009 for the all-to-all and 0.019 for the all-to-DMN matrix (see methods for the calculation of the SNR). Notice that SNR is simply one possible manner to calculate separability between two probability distributions. The fact that the distribution of strength values in Fig. 2B for the group of MOF patients was shifted to the right as compared to the one for HC, indicated that the DMN hyper-connected for the group of MOF patients as compared with HC. Importantly, when the SNR was calculated only over significant regions of the hyper-connectivity map, the increase in the SNR was from 0.013 to 0.157, indicating that a stronger statistical significance in the group comparison corresponded to a higher SNR.
Fig. 2.
Rectangular rather square FC matrices for assessing differences in functional connectivity. A: (left) Computation of node strength brain maps from the all-to-all FC matrices for both groups HC and MOF. (right) Probability distributions of strength obtained from the all-to-all FC matrix of the two groups, HC (blue) and MOF (red). Means and standard deviations are shown for the two groups, together with the signal to noise ratio (SNR). B: The same as in A, but for the all-to-DMN FC matrix. When compared A vs B, notice that the separability of the two distributions –measured by the SNR–increased from 0.009 in panel C to 0.019 in panel D, a ratio increment of 311%, and therefore, clarifying the quantitative advantage of using the all-to-DMN FC matrix rather than the all-to-all for performing group comparison. In addition, the distribution of the MOF patients shifted to the right, indicating hyper-connectivity. For both panels A and B, the dark red areas in the distributions represent the intersection between the blue and red distributions.
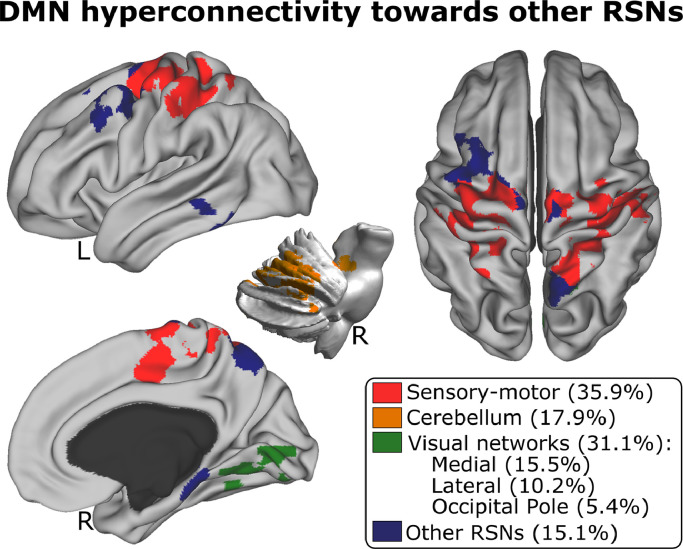
For the contrast [1 −1] (MOF > HC) the only FC matrix that provided significant differences was all-to-DMN. Fig. 3 illustrates the patterns of DMN hyper-connectivity towards three different RSNs: sensory-motor, cerebellum, and visual networks (the precise anatomical representation and cluster statistics of the significant brain maps are provided in Table S1). The contrast [−1 1] (MOF < HC) did not provide any significant differences after multiple comparisons for any class of FC matrix, indicating that no significant hypo-connection was found for any of the RSNs.
Fig. 3.
DMN hyper-connected towards major resting state networks. Significant maps resulting from group comparison with the contrast MOF type="Other"> HC using the all-to-DMN FC matrices. The resulting significant maps intersect with the following RSNs: sensory-motor, cerebellum, medial visual, lateral visual, occipital pole, and other RSNs. Percentage overlaps between hyper-connectivity maps and RSNs are indicated in brackets. The alternative contrast, MOF < HC connectivity did not show any significant voxel.
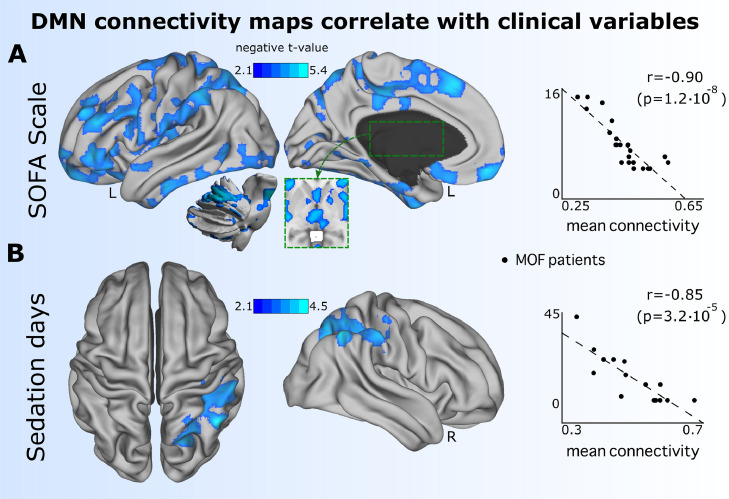
The strength maps obtained from the all-to-DMN matrix were associated with different clinical variables collected in the group of MOF patients: MMSE, SOFA, mean blood pressure, mean arterial partial oxygen pressure (pO2), and days of sedation (Table 1). The only two variables that had a significant association after multiple comparisons were SOFA and days of sedation (Fig. 4). The strength maps had a negative association with the SOFA scale (r = −0.90, p-value = 1.2 × 10–8), indicating that most severe patients during ICU stay had at six months after ICU discharge less connectivity of the DMN towards the following networks: sensory-motor, lateral visual, executive-control, auditory, and cerebellum (the anatomical representation and cluster statistics of the significant brain maps are provided in Table S2). Similarly, strength maps of all-to-DMN connectivity maps had a negative association with days of sedation (r = −0.85, p-value = 3.2 × 10–5), mainly in areas connecting the DMN with the frontoparietal network (Table S2).
Fig. 4.
Significant association between DMN connectivity patterns six months after ICU discharge and clinical scales during ICU stay. Maps of all-to-DMN FC values had a negative association with A: Worst SOFA maintained during 48 h in the ICU stay and B: Sedation days during ICU stay, indicating that the DMN connected less towards the brain areas plotted in blue for those patients who had more severe MOF (measured by SOFA) and had more days of sedation. To calculate the Spearman correlation (r) from the two scatter plots (right), it was only considered the mean t-value over the voxels which had associated p-values lower than the 5th percentile, ensuring a high value of correlation as compared to the situation of averaging the t-values over all voxels within the significant map (colored in blue).
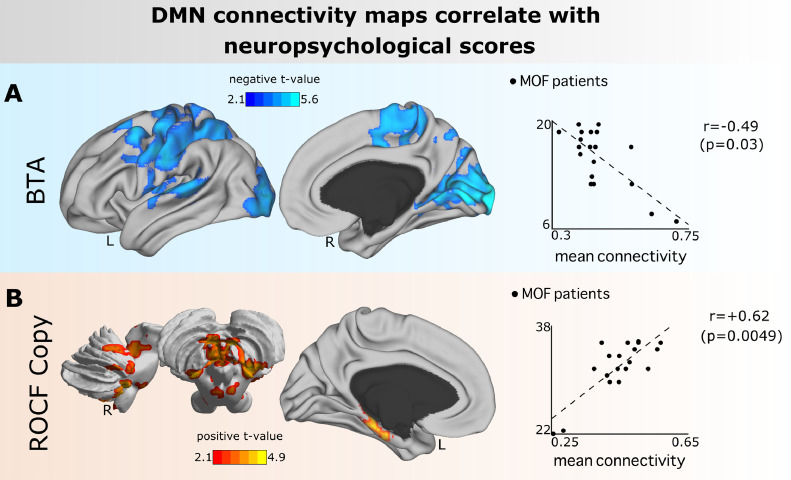
Lastly, the association of strength maps obtained from all-to-DMN connectivity matrices was assessed with each of the nine different neuropsychological tests. Fig. 5 illustrates the results for BTA (measuring attention) and ROCF-Copy (measuring visual perception, visual-spatial constructional ability, and visual memory), which were the only two tests providing a significant association, which was: 1. Negative for BTA (r = −0.49, p-value = 0.03), indicating that patients who performed better in BTA had less connectivity from DMN towards the following networks auditory, frontoparietal, lateral visual, medial visual, and sensory-motor networks (Table S3), 2. Positive for ROCF-Copy (r = +0.62, p-value = 0.0049), indicating that patients who performed better in ROCF-Copy had increased connectivity from DMN to the cerebellar network (Table S3).
Fig. 5.
Significant association between DMN connectivity patterns and neuropsychological scores (both measured at six months after ICU discharge). A: Maps of all-to-DMN FC values had a negative association with BTA (measuring attention), indicating that the DMN connected less towards the brain areas plotted in blue for those patients who performed better in attention (as measured by BTA). B: Maps of all-to-DMN FC values had a positive association with ROCF-Copy (measuring visuospatial integration), indicating that the DMN connected more towards the brain areas plotted in red for those patients who performed better in visuospatial integration (as measured by ROCF-Copy). Similar to Fig. 4, the Spearman correlation (r) from the two scatter plots (right) where calculated between the mean t-values over the voxels that had p-values lower than the 5th percentile.
4. Discussion
Multiorgan failure (MOF) is a progressive disorder triggered by a life-threatening insult that independently on the etiology of the injury has an associated high mortality rate. Despite ICU physicians have been mainly focused on patient's survival during ICU stay, approximately half of the patients who overcome MOF evince post-intensive care sequelae (Van Loey and Van Son, 2003) even for several years after the MOF insult (Pandharipande et al., 2013), having an impact on physical functional, cognitive, and psychiatric levels (Ehlenbach, 2010; Iwashyna et al., 2010; Latronico and Bolton, 2011; Wolters et al., 2013). Therefore, albeit it exists the need for improving patient's intervention during ICU for a better outcome, little is known about the neural mechanisms of the brain-related alterations in the long-term in MOF patients.
In this study, 22 patients who suffered MOF were recruited six months after ICU discharge and followed a complete MRI session and a comprehensive neuropsychological evaluation. The results have been compared with those obtained from an age-sex-education matched group of 22 HC. The study has focused on MOF patients with no structural brain damage on T1 MRI images, neither on qualitative inspection nor in quantitative (VBM) comparison with HC. However, the results show that MOF patients have clear functional connectivity abnormalities on resting-state brain MRI obtained six months after discharge from ICU, showing a hyper-connectivity pattern of DMN with other key brain networks such as sensory-motor, cerebellum, and visual networks. Moreover, the observed abnormal hyper-connectivity pattern of DMN was significantly associated with the severity of MOF during ICU stay and with worse scores in attention and visuospatial integration domain. To our knowledge, this is the first time that functional integrity of the MOF patients’ brains has been evaluated with advanced functional connectivity techniques. More importantly, these findings suggest that the study of brain functional connectivity with resting-state functional MRI might be a potential biomarker to predict cognitive outcome in MOF patients right after ICU discharge. Hyper-connectivity of the DMN might indicate a network adaptation mechanism for brain-damage compensation. Of crucial importance, hyper-connectivity of the DMN is not specific to MOF as it has been shown to occur in the onset of a plethora of pathologies, for instance, after concussion (Abbas et al., 2015), in the early stage of Alzheimer's disease (Schultz et al., 2017), in brain tumor survivors (Chen et al., 2016), deficit of consciousness (Di Perri et al., 2018), cancer related cognitive impairment (Apple et al., 2018), autism (Washington et al., 2014), and behavioral disorders such as anorexia (Cowdrey et al., 2014).
The strength maps obtained from the all-to-DMN connectivity matrix had a negative association with the severity scale of the multiorgan failure (SOFA), indicating that patients who had more severe MOF during ICU stay had less connectivity at six months after ICU discharge from the DMN towards many different networks such as sensory-motor, lateral visual, executive-control, auditory, and cerebellum. Similarly, less connectivity from the DMN towards the frontoparietal network was associated with the clinical variable of days of sedation, which likely reflects that the two variables (days of sedation and SOFA) have high statistical dependence between each other (r = 0.61, p-value = 0.012), showing that indeed the more severe patients made use of more days of sedation in their intervention.
The association of MOF severity and functional connectivity patterns of the DMN at six months after ICU discharge, even when no apparent brain structural damage exists in these patients, is somehow proving evidence related to the main study's hypothesis, namely, that functional connectivity may account for the brain alterations in these patients in the long-term. Whether the changes in the connectivity patterns found in the DMN reflect a negative (damage) or a positive effect (behavioral compensation) is difficult to confirm, and a longitudinal follow-up of this population is needed to fully assess this question.
When assessing cognitive functioning in MOF patients at six months after ICU discharge, no group-differences were found between MOF and HC in any of the neuropsychological tests, although the patients generally performed worse in all cognitive domains. The worse performance was found in learning and memory (as measured by the test HVLT-R Delayed) and in the speed of information processing and attention (measured by the tests Stroop Color and BTA). However, the brain maps of functional DMN connectivity to the rest of the brain did have a significant association with tests measuring visuospatial memory (ROCF-Copy) and attention (BTA). Although the effect size in these tests was not small (respectively, 0.21 and 0.5), the group differences were not significant, and therefore, the DMN connectivity patterns might indicate somehow anticipation or delay concerning the cognitive impairment, but to fully clarify this hypothesis, the follow-up of these patients is needed.
Very striking, the found patterns of functional connectivity of the DMN at six months after ICU discharge had an association with some severity scales during the ICU stay, but this did not happen when looking to associations between such ICU severity scores and neuropsychological parameters obtained also six months after ICU discharge. Therefore, our findings indicate that DMN connectivity might work as a biomarker of the onset of future cognitive deficits on these patients.
4.1. Methodological considerations and limitations
It should be noted that the limited sample size (N = 22 per group) and the short follow-up time in this study might bias the differences observed between MOF patients and HC. Moreover, restricting the participation to patients with an acceptable overall cognitive status (MMSE ≥ 23) led to the exclusion of those with more severe cognitive impairment (Folstein et al., 1975), thus eliminating possible comorbid factors that might affect the cognitive disability of MOF patients. Similarly, recruited MOF patients had no lesions detected in T1, which guaranteed that the cognitive impairment driven by structural lesions were also excluded from our study. Future studies with longer-term neuroimaging and neuropsychological data are needed to fully characterize whether or not our results might provide biomarkers for the cognitive deficits in MOF patients.
In this study the MOF causes were similar to previous studies assessing cognitive dysfunction in this systemic pathology (Pandharipande et al., 2013). However, there is also some heterogeneity among the MOF patients in terms of the cause for ICU admission, which could also have influenced the results obtained. Future studies should include more homogenous groups of patients with MOF in order to determine similarities and differences with the findings of this study.
For assessing the movement artifacts, scrubbing was performed for removing frames with FD greater than 0.5 together with one frame before and two frames after, which is a more conservative strategy ensuring good artifacts removal (Power et al., 2014). Our choice of 0.5 removed about 5% of the total number of scans in the MOF group, which is an appropriate number. After all the frames satisfying removal criteria were discarded, the shortest sequence in our population was equal to 5 min and 30 s, large enough for resting analyses. For the HC group, a total of 11 of 22 participants had discarded frames, with a mean of 13 removed frames per subject. For the HC participant with the highest movement, a total of 53 scans were removed (24.8% of the total) resulting in a sequence of 5 min 38 seg. For the group of MOF, a total of 11 of 22 participants had discarded frames, with a mean of 25 removed frames per subject. For the MOF patient with the highest movement, a total of 57 scans were removed (26.6% of the total) resulting in a sequence of 5 min 30 seg. After scrubbing, the two groups did not have significant differences in movement amount, as measured by a t-test in the number of discarded frames (p-value = 0.20).
Our statement of MOF patients not having brain structural damage is based on the following points: 1. Voxel-based morphometry did not provide significant group differences, 2. A clinical neuroradiologist (AC) inspected all MOF structural (T1) and functional (EPI) images, and after visual inspection did not find any lesion. Notice that hemorrhagic lesions (for instance) can be detected in EPI images. Similarly, T1 images allow detecting large parenchymatous lesions and none of our patients had any of these lesions. Unfortunately, FLAIR or T2 images were not acquired, so other classes of lesions detected from other structural sequences cannot be performed.
The all-to-DMN matrices were calculated using the RSNs templates provided by (Smith et al., 2009). Alternatively, it is possible to obtain ICA-derived time courses for each of the RSNs and calculate FC matrices from the rest of the brain to each of the networks. Figure S2 illustrates the two strategies. For the situation of having a good match between the template (DMN-T) and the spatial ICA-derived component (DMN-ICA), the two strategies are similar to each other (left column at panel B). However, when the spatial component does not match well the template, the two methods diverge (right column at panel B). By comparing panels A and B in figure S2, our strategy still captures the DMN connectivity pattern with itself, and this is not happening for the alternative of using ICA-derived time series.
5. Conclusion
It is known that MOF could cause some cognitive deficits, even to the levels seen in mild Alzheimer's disease. Nevertheless, it remains unclear what changes underlie such problems. For this reason, in this study, functional magnetic resonance imaging was performed on individuals that had suffered this pathology. Despite the absence of brain structural damage, some alterations in the pattern of functional connectivity were found. In particular, functional neuroimaging in the default mode network (DMN) showed hyper-connectivity towards sensory-motor, cerebellum, and visual networks. DMN connectivity patterns were significantly associated with the severity scales of MOF during ICU stay and with the neuropsychological outcome of patients (involving attention and visuospatial integration) six months after ICU discharge. To the best of our knowledge, this is the first study providing evidence for the functional impairments in brain networks in patients six months after MOF, although the precise neural mechanisms driving these functional, neurological, and cognitive deficits after the physiological insult are still unknown. Future studies should investigate the long-term consequences of these findings.
Author contributions
V.B., I.Gar. and F.L. recruited the participants; A.C.Z. collected the MRI data; D.R., D.R.U. and I.B.S. performed the neuropsychological evaluation, A.J.M. processed the MRI data; A.J.M., I.D., J.R., S.S., I.Gab. and J.M.C. designed and supervised the imaging and statistical analyses; A.J.M. performed the analyses; A.J.M. and J.M.C. made the figures; J.M.C. and J.C.A.L. supervised the research; all the authors wrote the manuscript and agreed with its submission; J.C.A.L and J.M.C. had equal last-author contribution.
Declaration of Competing Interest
None.
Acknowledgments
This research was founded by Ministerio Economia, Industria y Competitividad, Spain and FEDER (grant no. DPI2016-79874-R) to JC and JCAL. ID's time was founded by the Department of Education of the Basque Country, postdoctoral program. JR's time was founded by the Ministry of Education, Language Policy and Culture (Basque Government). JMC's time was founded by Ikerbasque and the Department of Economic Development and Infrastructure of the Basque Country, Elkartek Program (grant no. KK-2018/00032). JCAL's time was founded by Ikerbasque and Fundacion Mutua Madrileña (grant no. AP169812018). IG's time was founded by the Instituto de Salud Carlos III for a Juan Rodes (grant no. JR15/00008) co-funded by the European Regional Development Fund/European Social Fund ‘Investing in Your Future’. AJM's time was partly founded by Euskampus Fundazioa.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102137.
Appendix. Supplementary materials
References
- Abbas K., Shenk T.E., Poole V.N., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M., Robinson M.E. Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connect. 2015;5:91–101. doi: 10.1089/brain.2014.0279. [DOI] [PubMed] [Google Scholar]
- Apple A.C., Schroeder M.P., Ryals A.J., Wagner L.I., Cella D., Shih P.-A., Reilly J., Penedo F.J., Voss J.L., Wang L. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage Clinical. 2018;20:110–118. doi: 10.1016/j.nicl.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R.H.B., Schretlen D., Groninger L., Brandt J. The Hopkins verbal learning test-revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- Binnewijzend M.A.A., Schoonheim M.M., Sanz-Arigita E., Wink A.M., van der Flier W.M., Tolboom N., Adriaanse S.M., Damoiseaux J.S., Scheltens P., van Berckel B.N.M., Barkhof F. Resting-state fMRI changes in alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2012;33:2018–2028. doi: 10.1016/j.neurobiolaging.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Bonifazi P., Erramuzpe A., Diez I., Gabilondo I., Boisgontier M.P., Pauwels L., Stramaglia S., Swinnen S.P., Cortes J.M. Structure-function multi-scale connectomics reveals a major role of the fronto-striato-thalamic circuit in brain aging. Hum. Brain Mapp. 2018;39:4663–4677. doi: 10.1002/hbm.24312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino-Pontes B., Diez I., Jimenez-Marin A., Rasero J., Erramuzpe A., Bonifazi P., Stramaglia S., Swinnen S., Cortes J. Interaction information along lifespan of the resting brain dynamics reveals a major redundant role of the default mode network. Entropy. 2018;20:742. doi: 10.3390/e20100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Wang L., King T.Z., Mao H. Increased frontal functional networks in adult survivors of childhood brain tumors. Neuroimage Clin. 2016;11:339–346. doi: 10.1016/j.nicl.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., Carew J.D., Turski P.A., Moritz C.H., Quigley M.A., Meyerand M.E. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am. J. Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cortes J.M., Greve A., Barrett A.B., van Rossum M.C.W. Dynamics and robustness of familiarity memory. Neural Comput. 2010;22:448–466. doi: 10.1162/neco.2009.12-08-921. [DOI] [PubMed] [Google Scholar]
- Cowdrey F.A., Filippini N., Park R.J., Smith S.M., McCabe C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum. Brain Mapp. 2014;35:483–491. doi: 10.1002/hbm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Perri C., Amico E., Heine L., Annen J., Martial C., Larroque S.K., Soddu A., Marinazzo D., Laureys S. Multifaceted brain networks reconfiguration in disorders of consciousness uncovered by co-activation patterns: dynamic connectivity in disorders of consciousness. Hum. Brain Mapp. 2018;39:89–103. doi: 10.1002/hbm.23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez I., Drijkoningen D., Stramaglia S., Bonifazi P., Marinazzo D., Gooijers J., Swinnen S.P., Cortes J.M. Enhanced prefrontal functional-structural networks to support postural control deficits after traumatic brain injury in a pediatric population. Netw. Neurosci. 2017;1:116–142. doi: 10.1162/NETN_a_00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez I., Erramuzpe A., Escudero I., Mateos B., Cabrera A., Marinazzo D., Sanz-Arigita E.J., Stramaglia S., Cortes Diaz J.M., for the Alzheimer’s Disease Neuroimaging Initiative Information flow between resting-state networks. Brain Connect. 2015;5:554–564. doi: 10.1089/brain.2014.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J., James S., Voets N., Watkins K., Matthews P.M., James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Ehlenbach W.J. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely E.W., Shintani A., Truman B., Speroff T., Gordon S.M., Harrell F.E., Inouye S.K., Bernard G.R., Dittus R.S. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- Ferreira F.L., Bota D.P., Bross A., Mélot C., Vincent J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Girard, T.D., Jackson, J.C., Pandharipande, P.P., Pun, B.T., Thompson, J.L., Shintani, A.K., Gordon, S.M., Canonico, A.E., Dittus, R.S., Bernard, G.R., Wesley Ely, E., 2010. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness: critical care medicine38, 1513–1520. 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed]
- Golden C.J. Manual. Tea Ediciones; S.A: 2010. Stroop, Test de Colores y Palabras. [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther M.L., Morandi A., Krauskopf E., Pandharipande P., Girard T.D., Jackson J.C., Thompson J., Shintani A.K., Geevarghese S., Miller R.R., Canonico A., Merkle K., Cannistraci C.J., Rogers B.P., Gatenby J.C., Heckers S., Gore J.C., Hopkins R.O., Ely E.W. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the visions cohort magnetic resonance imaging study*. Critic. Care Med. 2012;40:2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.E., Trzeciak S., Kline J.A. The sequential organ failure assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit. Care Med. 2009;37:1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S. Editorial Medica Paramericana; S.A: 2005. Test de Vocabulario de Boston. [Google Scholar]
- Karbasforoushan H., Woodward N.D. Resting-state networks in schizophrenia. Curr. Top. Med. Chem. 2012;12:2404–2414. doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- Latronico N., Bolton C.F. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. The Lancet Neurol. 2011;10:931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- Li S., Ma X., Huang R., Li M., Tian J., Wen H., Lin C., Wang T., Zhan W., Fang J., Jiang G. Abnormal degree centrality in neurologically asymptomatic patients with end-stage renal disease: a resting-state fMRI study. Clinic. Neurophysiol. 2016;127:602–609. doi: 10.1016/j.clinph.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Li S.-J., Li Z., Wu G., Zhang M.-J., Franczak M., Antuono P.G. Alzheimer disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Liao W., Zhang Z., Pan Z., Mantini D., Ding J., Duan X., Luo C., Lu G., Chen H. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS ONE. 2010;5:e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.C. National Institutes of Health; 2001. The Multiple Organ Dysfunction syndrome, in: Surgical Treatment: Evidence-Based and Problem-Oriented. A Service of the National Library of Medicine; pp. 780–785. [Google Scholar]
- Needham D.M., Davidson J., Cohen H., Hopkins R.O., Weinert C., Wunsch H., Zawistowski C., Bemis-Dougherty A., Berney S.C., Bienvenu O.J., Brady S.L., Brodsky M.B., Denehy L., Elliott D., Flatley C., Harabin A.L., Jones C., Louis D., Meltzer W., Muldoon S.R., Palmer J.B., Perme C., Robinson M., Schmidt D.M., Scruth E., Spill G.R., Storey C.P., Render M., Votto J., Harvey M.A. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholdersʼ conference*. Critic. Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- Olabarrieta-Landa L., Torre E.L., Lopez-Mugartza J.C., Bialystok E., Arango-Lasprilla J.C. Verbal fluency tests: developing a new model of administration and scoring for Spanish language. NeuroRehabilitation. 2017;41:539–565. doi: 10.3233/NRE-162102. [DOI] [PubMed] [Google Scholar]
- Pandharipande P.P., Girard T.D., Jackson J.C., Morandi A., Thompson J.L., Pun B.T., Brummel N.E., Hughes C.G., Vasilevskis E.E., Shintani A.K., Moons K.G., Geevarghese S.K., Canonico A., Hopkins R.O., Bernard G.R., Dittus R.S., Ely E.W. Long-Term cognitive impairment after critical illness. N. Engl. J. Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasero J., Pellicoro M., Angelini L., Cortes J.M., Marinazzo D., Stramaglia S. Consensus clustering approach to group brain connectivity matrices. Netw. Neurosci. 2017;1:242–253. doi: 10.1162/NETN_a_00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan, R.M., Wolfson, D., 1985. The Halstead–Reitan neuropsycholgical test battery: therapy and clinical interpretation. Neuropsychological Press.
- Rey A. Tea Ediciones; S.A: 2009. FTest de Copia y Reproducción de una Figura Compleja. [Google Scholar]
- Rombouts S.A.R.B., Barkhof F., Goekoop R., Stam C.J., Scheltens P. Altered resting state networks in mild cognitive impairment and mild alzheimer’s disease: an fMRI study. Hum. Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D. Brief test of attention professional manual. Psychol. Assess. Resour. 1997 [Google Scholar]
- Schretlen D., Bobholz J., Brandt J. Development and psychometric properties of the brief test of attention. Clin. Neuropsychol. 1996;10:80–89. doi: 10.1080/13854049608406666. [DOI] [Google Scholar]
- Schultz A.P., Chhatwal J.P., Hedden T., Mormino E.C., Hanseeuw B.J., Sepulcre J., Huijbers W., LaPoint M., Buckley R.F., Johnson K.A., Sperling R.A. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and Tau in clinically normal individuals. J. Neurosci. 2017;37:4323–4331. doi: 10.1523/JNEUROSCI.3263-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. TEA Ediciones; S.A: 2013. Test de simbolos y digitos. [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, E., Sherman, E., Spreen, O., 2006. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford University Press.
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Loey N.E.E., Van Son M.J.M. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am. J. Clin. Dermatol. 2003;4:245–272. doi: 10.2165/00128071-200304040-00004. [DOI] [PubMed] [Google Scholar]
- Washington S.D., Gordon E.M., Brar J., Warburton S., Sawyer A.T., Wolfe A., Mease-Ference E.R., Girton L., Hailu A., Mbwana J., Gaillard W.D., Kalbfleisch M.L., VanMeter J.W. Dysmaturation of the default mode network in autism: dysmaturation of default network in autism. Hum. Brain Mapp. 2014;35:1284–1296. doi: 10.1002/hbm.22252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters A.E., Slooter A.J.C., van der Kooi A.W., van Dijk D. Cognitive impairment after intensive care unit admission: a systematic review. Intensive Care Med. 2013;39:376–386. doi: 10.1007/s00134-012-2784-9. [DOI] [PubMed] [Google Scholar]
- Woodward N.D., Rogers B., Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr. Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.