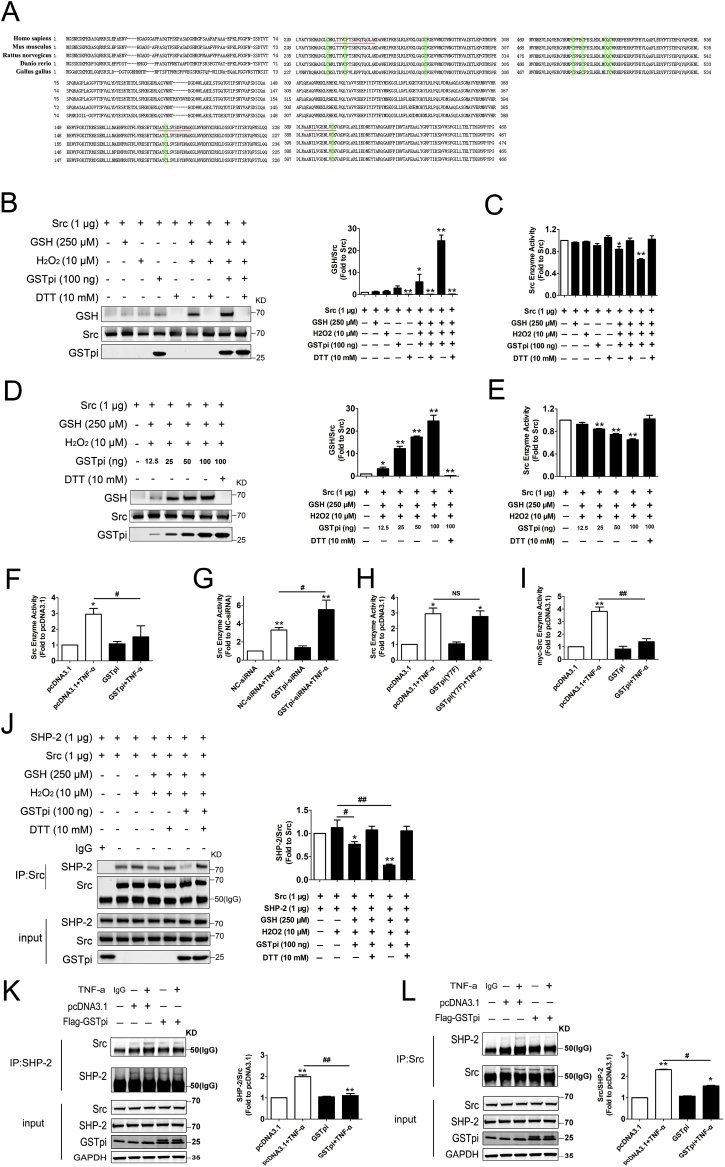
Fig. 6.
In Vitro S-glutathionylation of Src. (A) Amino acid sequence alignments of full-length Src. Green highlight, conserved cysteines in Src protein; underline, cysteine-containing peptides detected in MS analysis. His-tag-purified Src was incubated with 10 μM H2O2, 250 μM glutathione and 100 ng GSTpi. (B) Immunoblots of glutathione-modified human Src with glutathionylation antibody. Total protein loading was evaluated with Src -specific antibodies. (C) Src kinase assay of S-glutathionylated Src protein. His-tag-purified Src was incubated with 10 μM H2O2, 250 μM glutathione, different contents GSTpi. (D) Immunoblots of glutathione-modified human Src with glutathionylation antibody. Total protein loading was evaluated with Src -specific antibodies. (E) Src kinase assay of S-glutathionylated Src protein. (F–I) Src kinase assay of Flag-GSTpi, GSTpi-siRNA, Flag-GSTpi (Y7F) and myc-Src, Flag-GSTpi transfected HUVEC cells. (J) His-tag-purified Src was incubated with 10 μM H2O2, 250 μM glutathione, 100 ng GSTpi. Further co-immunoprecipitation of SHP-2 and Src was performed, and relative SHP-2 levels were measured and calculated (IP results come from different blots with same samples). (K–L) HUVECs were transfected with pcDNA3.1 or Flag-GSTpi and further treated with TNF-α (50 ng/mL) for 30 min. Co-immunoprecipitation of SHP-2 and Src was performed, and relative Src and SHP-2 levels were measured and calculated (IP results come from different blots with same samples. *, compared with the pcDNA3.1 group; #, compared with the pcDNA3.1 + TNF-α group). Data are expressed as means ± SDs (n = 3). Data were analyzed by unpaired Student's t-tests. * or #, P < 0.05; ** or ##, P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)