Figure 2.
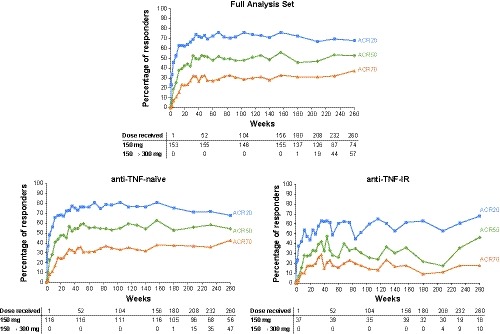
ACR20/50/70 responses up to 5 years in the secukinumab 150‐mg group. To show the full 5‐year efficacy, results are shown for the group of patients who were originally randomized to secukinumab 150 mg during the core study and entered the extension study in the secukinumab 150 mg group; patients who switched from placebo to secukinumab 150 mg at weeks 16 and 24 are not included in these figures. ACR 20/50/70, American College of Rheumatology criteria for 20%/50%/70% improvement in disease activity.
